Summary: University of Michigan researchers have developed an artificial photosynthesis system that efficiently converts CO2 into ethylene, outperforming previous systems in efficiency, yield, and longevity.
Estimated reading time: 5 minutes
Scientists at the University of Michigan have made a significant leap in artificial photosynthesis technology. Their new system chains carbon atoms together to produce ethylene from carbon dioxide with unprecedented efficiency and stability. This advancement could pave the way for more sustainable production of plastics and, potentially, liquid fuels.
A New Approach to Carbon Capture and Utilization
The research team, led by Zetian Mi, professor of electrical and computer engineering, has created a device that mimics natural photosynthesis to convert CO2 into useful hydrocarbons. Their system focuses on producing ethylene, a crucial ingredient in many plastics and one of the most produced organic compounds worldwide.
“The performance, or the activity and stability, is about five to six times better than what is typically reported for solar energy or light-driven carbon dioxide reduction to ethylene,” Mi stated. This improvement marks a substantial step forward in the field of artificial photosynthesis.
How the System Works
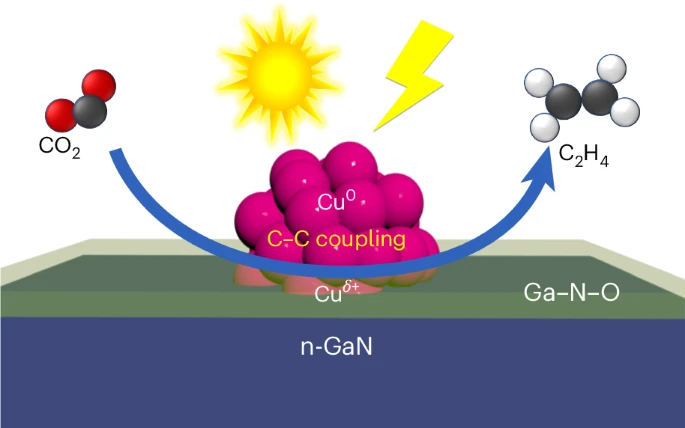
The device’s core consists of a forest of gallium nitride nanowires grown on a silicon base. These nanowires, each just 50 nanometers wide, are dotted with copper clusters of about 30 atoms each. When submerged in CO2-enriched water and exposed to sunlight, the system initiates a complex chemical reaction.
Light energy frees electrons that split water molecules near the nanowires’ surface, creating hydrogen. The copper clusters then facilitate the binding of carbon from CO2 with this hydrogen, ultimately forming ethylene. Remarkably, 61% of the free electrons generated contribute to this ethylene-producing reaction.
Unprecedented Efficiency and Longevity
What sets this system apart is not just its efficiency but its longevity. While a competing silver-copper catalyst achieved similar efficiency, it could only function for a few hours. In contrast, the Michigan team’s device ran for 116 hours without slowdown, and similar devices have operated for up to 3,000 hours.
The system’s durability is partly due to a synergistic relationship between the gallium nitride and the water-splitting process. Oxygen produced during the reaction improves the catalyst and enables a self-healing process, contributing to the device’s longevity.
Implications and Future Directions
This breakthrough has significant implications for sustainable manufacturing and energy production. Ethylene, typically produced from oil and gas under high temperatures and pressures, could now be made using captured CO2, potentially reducing industrial carbon emissions.
Bingxing Zhang, the study’s first author, looks ahead: “In the future, we want to produce some other multicarbon compounds such as propanol with three carbons or liquid products.” The ultimate goal is to create liquid fuels, which could revolutionize transportation by making existing technologies more sustainable.
Challenges and Questions
While the results are promising, several questions remain. The team plans to explore the limits of the device’s longevity in future work. Additionally, scaling up the technology for industrial use and ensuring its economic viability will be crucial next steps.
Some may wonder about the energy input required for this process and whether it truly results in a net reduction of CO2. The researchers emphasize that the system uses sunlight as its primary energy source, potentially making it a sustainable alternative to current ethylene production methods.
As we continue to grapple with climate change and seek sustainable alternatives to fossil fuels, innovations like this artificial photosynthesis system offer hope. By turning a greenhouse gas into a valuable product, we might not only reduce emissions but also create a circular economy where waste becomes a resource.
Quiz: Test Your Knowledge
- What is the main product of the artificial photosynthesis system described in the article? a) Methane b) Ethanol c) Ethylene d) Propanol
- How long did the device run without slowing down in the reported experiment? a) 16 hours b) 116 hours c) 1,160 hours d) 3,000 hours
- What percentage of free electrons generated by the semiconductors contributed to the ethylene-producing reaction? a) 30% b) 45% c) 61% d) 75%
Answers:
- c) Ethylene
- b) 116 hours
- c) 61%
For further reading:
- Artificial Photosynthesis: Creating Fuel from Sunlight
- Carbon Capture and Utilization Technologies
- The Global Status of CCS: 2023
Glossary of Terms:
- Artificial Photosynthesis: A chemical process that mimics natural photosynthesis to convert sunlight, water, and carbon dioxide into useful products.
- Ethylene: A hydrocarbon compound (C2H4) widely used in the production of plastics and other industrial chemicals.
- Nanowires: Extremely thin wires with diameters on the nanometer scale, used in various nanotechnology applications.
- Catalyst: A substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change.
- Carbon Capture and Utilization (CCU): Technologies that capture carbon dioxide emissions and convert them into useful products.
Enjoy this story? Get our newsletter! https://scienceblog.substack.com/
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!