Researchers have discovered that the widely prescribed Parkinson’s drug entacapone disrupts the gut microbiome by inducing iron deficiency, favoring the growth of potentially harmful bacteria like E. coli. The findings highlight the need to consider drug effects on gut health in treatment plans.
Published in Nature Microbiology | Estimated reading time: 6 minutes
Drug-Induced Iron Deficiency Alters Gut Microbial Communities
While drugs like antibiotics are well-known to affect the gut microbiome, researchers are increasingly recognizing that non-antibiotic medications, such as those targeting neurological conditions, can have profound effects on gut bacteria. A new study led by scientists at the University of Vienna found that entacapone, a drug used to treat Parkinson’s disease, induces iron deficiency in the gut, disrupting the microbial balance and encouraging the growth of pathogens like E. coli.
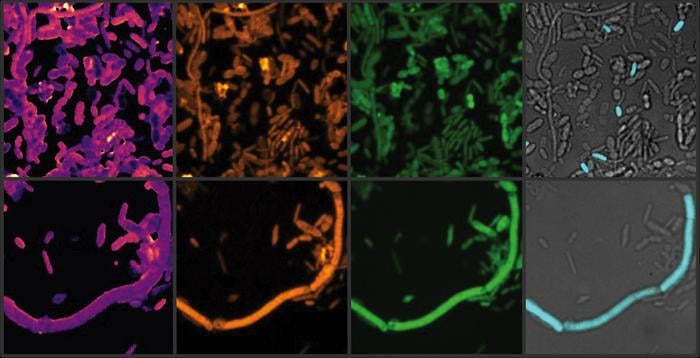
The study, published in Nature Microbiology, used advanced techniques such as heavy water labeling and Stimulated Raman Spectroscopy (SRS) to analyze how entacapone and loxapine (a schizophrenia medication) affect gut bacteria. Researchers incubated human fecal samples with these drugs and observed significant disruptions, particularly with entacapone. Lead author Fatima Pereira noted that the experiments revealed subtle but important changes in microbial activity that are often overlooked in traditional studies.
Mechanism Behind Microbiome Disruption
Entacapone’s microbiome-altering effects are linked to its ability to bind and sequester iron, a vital nutrient for many microbes. This iron starvation creates an environment where bacteria with efficient iron uptake systems, such as E. coli, can thrive. Adding iron to the samples reversed these effects, suggesting that entacapone-induced dysbiosis might be mitigated by addressing iron availability in the gut.
“By showing that entacapone induces iron deficiency, we uncovered a new mechanism of drug-induced gut dysbiosis,” explained Michael Wagner, a senior author of the study. The researchers highlighted that drugs with similar chemical structures, including other medications containing metal-binding catechol groups, might also affect the gut microbiome in this way.
Implications for Patient Care
The findings have broader implications for improving the safety and efficacy of drug treatments. Ensuring adequate iron delivery to the large intestine could help maintain microbiome balance while avoiding gastrointestinal side effects often seen in Parkinson’s patients. Future research will explore strategies to modify drug delivery to support gut health without compromising therapeutic effectiveness.
Glossary
- Microbiome: The community of microorganisms, including bacteria, fungi, and viruses, that live in and on the human body.
- Dysbiosis: An imbalance in the composition or function of the gut microbiome, often associated with health issues.
- Iron Starvation: A condition where microorganisms lack sufficient iron, an essential nutrient for their growth.
- Stimulated Raman Spectroscopy (SRS): A molecular imaging technique used to study chemical changes at high resolution.
- Enterobactin Siderophore: A molecule produced by bacteria like E. coli to efficiently capture and utilize iron.
Quiz
What is the primary finding of the study?
Entacapone disrupts the gut microbiome by inducing iron deficiency, favoring the growth of E. coli.
How does entacapone affect the gut microbiome?
By sequestering iron, which disrupts microbial balance and promotes the growth of iron-efficient bacteria.
What technique was used to analyze microbial activity?
Stimulated Raman Spectroscopy (SRS) combined with heavy water labeling.
What potential solution could mitigate entacapone’s side effects?
Providing targeted iron supplementation to the large intestine to reduce dysbiosis.
Enjoy this story? Subscribe to our newsletter at scienceblog.substack.com