Japanese scientists have developed an innovative system that could harness sunlight to split water into hydrogen and oxygen, potentially offering a sustainable pathway to renewable hydrogen fuel. The breakthrough comes from researchers at Shinshu University who have demonstrated the technology’s feasibility at an unprecedented scale.
Published in Frontiers in Science | Estimated reading time: 4 minutes
In a world seeking alternatives to fossil fuels, the ability to produce hydrogen from water using only sunlight could transform our energy landscape. While most hydrogen today comes from natural gas, researchers are making significant strides toward a cleaner approach using advanced photocatalytic materials and an innovative panel reactor system.
“Sunlight-driven water splitting using photocatalysts is an ideal technology for solar-to-chemical energy conversion and storage, and recent developments in photocatalytic materials and systems raise hopes for its realization,” explains Professor Kazunari Domen of Shinshu University, senior author of the study.
The technology relies on photocatalysts – materials that promote chemical reactions under light. Two approaches are being developed: a simpler one-step system that directly splits water into hydrogen and oxygen, and a more efficient two-step system using separate catalysts for hydrogen and oxygen production.
The research team has already achieved a significant milestone by operating a 100-square-meter reactor for three years. Remarkably, this real-world demonstration performed even better than laboratory tests. As Dr. Takashi Hisatomi notes, “In our system, using an ultraviolet-responsive photocatalyst, the solar energy conversion efficiency was about one and a half times higher under natural sunlight.”
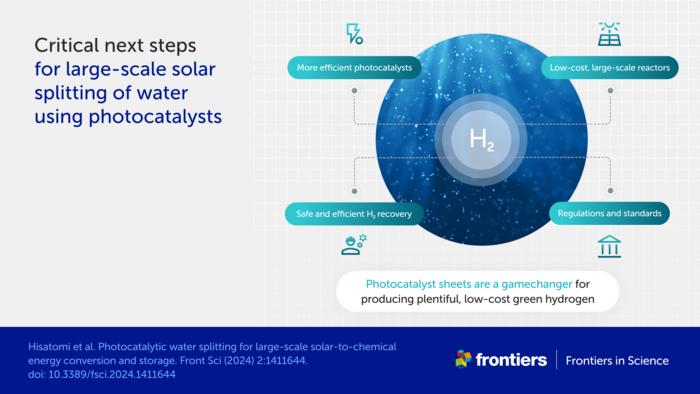
However, challenges remain before widespread adoption becomes feasible. Current efficiency rates hover around 1%, while practical implementation would require at least 5% efficiency. The researchers emphasize that achieving this goal will require continued development of more efficient photocatalysts and larger experimental reactors.
To accelerate progress, the team advocates for establishing safety regulations and efficiency standards, including an accreditation body and licensing system. These measures would help ensure safe technology development while standardized efficiency measurements would aid in identifying the most promising systems.
Glossary
- Photocatalyst
- A material that speeds up chemical reactions when exposed to light, similar to how plants use chlorophyll to harness sunlight for photosynthesis.
- Solar-to-Chemical Energy Conversion
- The process of converting sunlight into stored chemical energy, such as hydrogen fuel, that can be used later when needed.
- Energy Conversion Efficiency
- The percentage of incoming solar energy that is successfully converted into usable hydrogen fuel, a critical measure of the technology’s practical viability.
Test Your Knowledge
What is the current main source of hydrogen fuel production?
Most hydrogen is currently derived from natural gas, making it dependent on fossil fuels.
What scale did researchers achieve in their reactor demonstration?
The researchers operated a 100-square-meter reactor successfully for three years.
What efficiency level is needed for practical implementation of this technology?
The technology needs to achieve at least 5% efficiency for practical implementation, while current rates are around 1%.
How did the real-world reactor performance compare to laboratory tests, and what explains this difference?
The real-world performance was about 1.5 times better than laboratory tests because the system could use a larger fraction of UV light from natural sunlight compared to simulated light.
Enjoy this story? Subscribe to our newsletter at scienceblog.substack.com.