Researchers at the University of Rochester Medical Center and University of Nebraska Medical Center have received a $3.4 million grant from the National Institutes of Health to study an experimental drug combination that appears to rid white blood cells of HIV and keep the infection in check for long periods. While current HIV treatments involve pills that are taken daily, the experimental drugs’ long-lasting effects suggest the possibility of an HIV treatment that could be administered monthly, or perhaps a few times a year.
Each of the experimental HIV drugs has been in development for several years, one at Rochester and the other at Nebraska. But earlier this year they were tested together, in laboratory experiments using human immune cells and mice that were engineered to have a human immune system. The purpose of the experiments was to determine whether one drug might interfere with the effectiveness of the other. To the astonishment of the researchers, the opposite occurred: one drug boosted the effectiveness of the other.
Rochester’s drug was developed in an unexpected place – the laboratory of a pediatric neurologist. While HIV is thought of as a virus that attacks the immune system, it also attacks the brain, causing cognitive impairment in many patients. Harris A. Gelbard, M.D., Ph.D., director of UR’s Center for Neural Development and Disease, has spent his career studying HIV’s toll on the brain and looking for ways to intervene. His research led him to focus on an enzyme, called mixed lineage kinase type 3 (MLK3) that appears to play a key role in the damaging inflammation that occurs when HIV infects a cell.
Since 2001 Gelbard has led a long-term, NIH-funded research effort called a Research Program Project Grant that enabled him to collaborate with other researchers, including Stephen Dewhurst, Ph.D., chair of Microbiology and Immunology at Rochester and Val Goodfellow, Ph.D., of the San Diego biotech firm Califia Bio, Inc. The group designed and tested an experimental drug that would inactivate the MLK3 enzyme in hopes that it would dampen the body’s inflammatory response to an HIV infection, and in turn reduce its harmful effects on the brain.
The compound, called URMC-099, proved successful as an anti-inflammatory and neuroprotective agent in mouse experiments that were reported last year in the Journal of Neuroscience and Journal of Medicinal Chemistry, and Gelbard’s team began additional experiments necessary to receive FDA approval to begin human studies.
While the experiments were conducted in the lab, they were designed to mimic the real-world experience of HIV patients who would ultimately take the drug. Gelbard understood that any patient who would be prescribed URMC-099 to alleviate HIV’s harmful effects on the brain would also be taking another type of drug, called a protease inhibitor, which is a standard therapy to combat HIV’s effects on white blood cells. In order for URMC-099 to be safely tested in humans, researchers would first have to conduct tests to make sure that it wouldn’t weaken the effects of a protease inhibitor or cause other unexpected side effects when the two drugs were administered together.
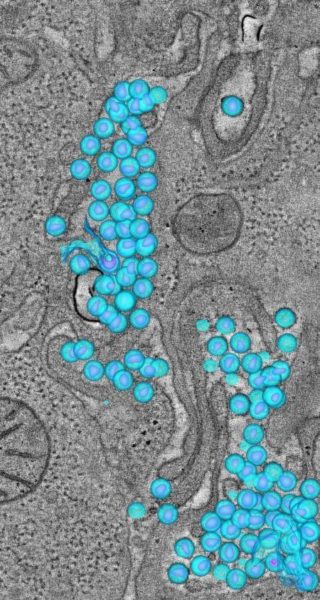
In one of those experiments, URMC-099 was tested in combination with an experimental protease inhibitor called nanoformulated ritonavir-boosted atazanavir, or nanoATV/r, which is being developed in the laboratory of Howard E. Gendelman at the University of Nebraska Medical Center. Gendelman and Gelbard, who have been collaborators since 1992, designed a set of experiments – using human immune cells grown in a lab and mice engineered to have a human immune system – to determine whether the drugs could be safely administered together.
The results stunned both researchers: URMC-099 appeared to dramatically boost the ability of nanoATV/r to rid white blood cells of HIV. It also appeared to slow the rate at which nanoATV/r was eliminated from white blood cells, thereby prolonging its therapeutic effect. And URMC-099 appeared to make it more difficult for the HIV virus to assemble inside white blood cells. Preliminary results from the experiments were presented at an international immunology meeting, the Conference on Retroviruses and Opportunistic Infections, in 2013.
“HIV researchers have suspected for many years that the MLK3 enzyme plays a fundamental role in the inflammatory process that’s triggered by an HIV infection,” said Gelbard. “Our original work showed that inactivating MLK3 had beneficial effects on the brain, but it now appears that the effects are more broad than that. MLK3 inactivation appears to produce beneficial effects in white blood cells that allowed this nanoformulated protease inhibitor to work much more effectively.”
The new NIH grant, led by Gelbard, will enable Gendelman’s and Gelbard’s labs to conduct additional experiments aimed at revealing the mechanisms by which URMC-099 boosts the effectiveness of nanoATV/r, and to assess whether the drug combination produces any toxic effects that might prevent it from being safely administered to people. If those tests are successful, Gelbard plans to file for FDA approval to begin clinical trials of URMC-099 in 2015.
Since 2001, Gelbard has received more than $20 million from the National Institutes of Health to fund his research into HIV’s effects on the brain and the role of the MLK3 enzyme, and the development of URMC-099.