Diagnosing HIV and other infectious diseases presents unique challenges in remote locations that lack electric power, refrigeration, and appropriately trained health care staff. To address these issues, researchers funded by the National Institutes of Health have developed a low-cost, electricity-free device capable of detecting the DNA of infectious pathogens, including HIV-1. The device uses a small scale chemical reaction, rather than electric power, to provide the heat needed to amplify and detect the DNA or RNA of pathogens present in blood samples obtained from potentially infected individuals.
“This highly creative technical solution brings sophisticated molecular diagnostics to underserved populations and represents a potentially groundbreaking advance in global health care for HIV as well as tuberculosis and malaria, which remain significant health challenges in many remote areas,” said Roderic Pettigrew, Ph.D., M.D., director of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) at NIH.
The work was performed by a team at the Seattle-based international non-profit PATH, led by Paul LaBarre, senior technical officer, and is reported in the Nov. 26 issue of PLOS ONE. The core technology they developed and continue to improve is a system known as NINA, for non-instrumental nucleic acid amplification. The goal is to expand access to accurate diagnostics wherever they are needed, especially those areas that lack reliable electricity.
Early on-site diagnosis allows immediate treatment
LaBarre explains the problem their research endeavors to address. “In low-resource settings, the lack of on-site molecular diagnostic testing is a barrier to the control of infectious diseases. The need to transport the samples from local sites to a distant central diagnostic facility results in delays, lost results, and increased costs.” One of the biggest problems, he says, is loss to follow-up, where individuals who have provided samples may fail to return to the local clinic, and therefore will not receive treatment if their test result indicates they are infected. Given these significant impediments to effective disease control, the goal of the NINA technology is to enable on-site point of care (POC) testing and subsequent treatment regardless of the available infrastructure.
A critical feature of the nucleic acid test is the ability to detect infection at very early stages. The currently available test sold over the counter is antibody-based, and cannot detect HIV until antibodies to the pathogen are produced by the body, which can take as long as several months after infection. The PATH method can detect HIV in the early stage of the disease, when the patient can be most infectious. Early detection is essential for POC medicine, where the goal is to diagnose infection and begin treatment in a single visit to the local clinic. For testing babies born to HIV-positive mothers, a nucleic acid-based test must be used because the mother’s antibodies in the baby’s blood can result in false positives.
Addressing the challenge one idea at a time
The amplification process involves extracting nucleic acids from an individual’s blood sample, mixing it with a nucleic acid segment from the pathogen of interest, and using constant temperature heat in a process that makes many copies of (amplifies) pathogen nucleic acids present in the blood sample. The results of the test are highly accurate and easily visualized with a simple dipstick that reveals a colored band indicating the presence of the pathogen nucleic acids.
LaBarre and his team are developing the NINA system using an inexpensive insulated thermos where the source of heat is the chemical reaction. The newest version of the incubator produces heat using magnesium iron alloy (MgFe). MgFe was chosen because it costs just $0.06 per reaction and can be supplied in a self-contained packet. To start the heat-producing reaction, a technician simply adds saline solution to the packet at the bottom of the thermos.
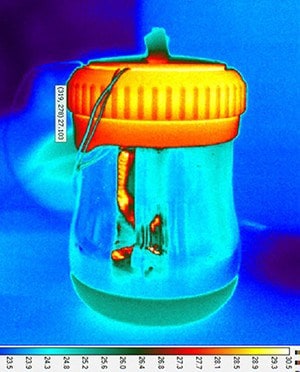
In this study, the researchers engineered each component of the incubator for maximum performance, ensuring that the amplification reaction that takes place in tiny test tubes maintains a constant temperature. To achieve this, the group identified a special compound that can store and regulate the heat created by the chemical reaction and can also be easily configured to the tube-holder design, guaranteeing uniform heating on each tube’s surface. When designing the main body of the device, the research team used a thermal imaging camera to assess the performance of inexpensive materials, and eventually chose a reusable thermos and cover that minimize system heat loss.
Another critical factor is the setting in which the incubator must operate. Although a sophisticated diagnostic laboratory would have equipment operating at room temperature in a controlled environment, the device must operate in extreme ambient temperatures. The reaction inside the incubator must maintain a temperature of 140 degrees Fahrenheit for one hour. Therefore, the research team checked the ability of the NINA incubator to function properly over a range of ambient temperatures. The device maintained the required 140 degrees when tested in environments ranging from 50 to 90 degrees.
The group demonstrated that their amplification system provides sensitive and repeatable detection of HIV-1 in just 80 minutes. They are now working to pair their amplification system with a simple technique for preparing nucleic acids from blood samples in the field. LaBarre explains: “To complete this low-resource setting diagnostic, one remaining need is the integration of a simple method for isolating nucleic acids from patient blood samples before amplification. Current methods are expensive and technically difficult. Fortunately, there are several methods we are testing that look promising.”
Identifying high-performance, yet inexpensive materials
Because the NINA system can quickly determine whether an individual has an infectious disease, it is a critical technology that will enable POC health services, which combine testing and treatment in a single visit. This is an essential step toward the control and eventual eradication of infectious diseases across regions of small, isolated villages.
This work was supported by NIH through the National Institute of Biomedical Imaging and Bioengineering under award number R01EB012641 and the National Institute of Allergy and Infectious Diseases under award number R01AI097038.