A new study by an international team of researchers shows for the first time that people may inherit some of the intestinal bacteria that cause Crohn’s disease and ulcerative colitis, collectively know as inflammatory bowel disease (IBD). The study, recently published in Genome Medicine, also confirmed that antibiotics could worsen the imbalance in the gut microbes.
About 1.6 million Americans suffer from Crohn’s disease or ulcerative colitis, according to the Crohn’s and Colitis Foundation of America. Understanding the causes of these diseases is another step toward prevention and treatment.
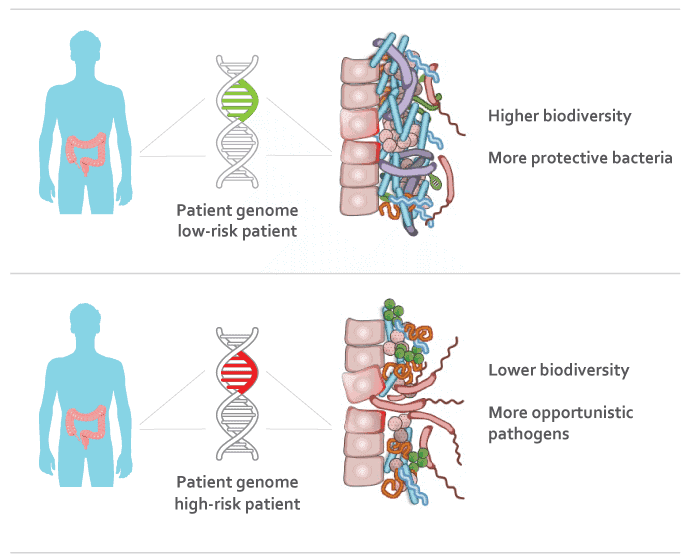
“The intestinal bacteria, or ‘gut microbiome,’ you develop at a very young age, can have a big impact on your health for the rest of your life,” said the study’s lead author Dan Knights, a University of Minnesota assistant professor in the Department of Computer Science and Engineering and the Biotechnology Institute. “We have found groups of genes that may play a role in shaping the development of imbalanced gut microbes.”
Knights and his colleagues at the University of Minnesota worked with collaborators at Harvard, MIT, University of Toronto and University Medical Center Groningen in one of the largest international studies of its kind. The researchers examined three independent cohorts of a total of 474 adults with IBD who live in Boston, Mass. (USA); Toronto, Ontario (Canada); and Groningen (Netherlands). Doctors and nurses in those locations collected samples of DNA from each human subject and the DNA of their intestinal bacteria over about a two-year period. The researchers looked at thousands of microbial species and human genes.
The results, which were replicated across two or more cohorts, showed that the human subjects’ DNA was linked to the bacteria in their intestines. Patients with IBD had lower biodiversity of bacteria and more opportunistic bacteria. This is an important step in developing drug treatments that target certain genes or certain products derived from the intestinal bacteria.
The intestinal community of bacteria is a complex system that is not only affected by genetics, but also by age, gender, medication and other factors. For example, this study confirmed that use of antibiotics is associated with a greater imbalance in the bacterial community in the intestines. Previous studies have shown links between human gut bacteria and increased risk of a wide variety of diseases including diabetes, autism, heart disease, and even some forms of cancer.
“In many cases we’re still learning how these bacteria influence our risk of disease, but understanding the human genetics component is a necessary step in unraveling the mystery,” Knights said.
The research was funded in part by the National Institutes of Health (NIH), the National Science Foundation, the Crohn’s and Colitis Foundation of America, and the Crohn’s and Colitis Foundation of Canada.
In addition to the University of Minnesota, authors of the study were from the Broad Institute of Harvard and MIT, Massachusetts General Hospital and Harvard Medical School, Mount Sinai Hospital in Toronto, University Medical Center Groningen in the Netherlands, University of Toronto, and the Harvard School of Public Health.
To read the full research study entitled, “Complex host genetics influence the microbiome in inflammatory bowel disease,” visit the Genome Medicine website.