UC Berkeley scientists have found the mechanism by which titanium, prized for its high strength-to-weight ratio and natural resistance to corrosion, becomes brittle with just a few extra atoms of oxygen.
The discovery, described in the Feb. 6 issue of the journal Science, has the potential to open the door to more practical, cost-effective uses of titanium in a broader range of applications. The popular silver-gray metal can already be found in high-end bicycles, laptops and human implants, among other products. But high-grade titanium with low levels of oxygen is hard to come by, and the expense of purifying the metal has prevented its wider use in applications for the construction, automotive and aerospace industries.
“If you could process titanium in a way that retained its optimal properties but at a cost comparable to aluminum, you would find uses in cars, trucks, aircraft and ships,” said study senior author Andrew Minor, an associate professor of materials science and engineering and faculty scientist at Lawrence Berkeley National Laboratory. “The high corrosion resistance and excellent specific properties of titanium are very attractive, and reducing the costs to the level of aluminum would make using the material a no-brainer.”
Minor led a research team from the department of materials science and engineering that focused on solving the long-standing mystery in metallurgy of how oxygen causes such a profound change in the characteristics of metals.
“Oxygen is like poison to titanium,” said Minor. “With more oxygen, the material gets harder and more susceptible to cracks, qualities that are not desirable for structural materials.”
A good structural material will have the right balance of ductility — the ability to bend in response to stress — and strength. Minor noted that glass is strong and hard, but not ductile, which is why that material is not used to build vehicles or bridges.
Minor added that while many metals have the potential to become brittle with oxygen, titanium is particularly sensitive to even tiny bits of the element. Grade 3 titanium is only 0.3 percent oxygen, yet it is one-third as tough as grade 1 titanium, which is 0.1 percent oxygen. Understanding how oxygen hardens titanium offers a target for research into control of the process, the study authors said.
The researchers subjected various grades of titanium samples to nanocompression tests and examined the resulting impact using advanced transmission electron microscopy techniques and quantum mechanical predictions of defect structures. They found that the interactions between oxygen and the crystalline defects, known as dislocations, that are characteristic of titanium were key to how the material hardened.
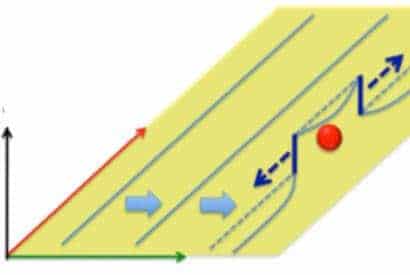
The researchers found that oxygen atoms acted like bumps in the road for the corkscrew-shaped dislocations found in titanium. “The mechanical shuffling that occurs as dislocations pop up and over those atomic bumps creates a domino effect of more dislocations,” said study co-author Daryl Chrzan, a professor of materials science and engineering who led the theoretical effort in the project. With increased oxygen, the titanium becomes more difficult to bend and therefore more susceptible to cracking, the researchers found.
A similar effect is seen by bending a paper clip until it breaks. The more the metal bends, the greater the number of dislocations. Dislocations interfere with the motion of other defects, making the paper clip more difficult to bend. Eventually, the number of dislocations is so high that the paper clip can no longer bend, and instead it breaks.
“Now that we know what it is about the oxygen found in inexpensive titanium that causes the material to harden, we can work on figuring out a way to process it to move oxygen atoms to a place where they don’t cause problems,” said study co-author Mark Asta, a professor of materials science and engineering.
Minor noted that this is already done in the semiconductor industry since oxygen and other impurities are also damaging to silicon-based microprocessors.
Other co-authors of the study included researchers from the Berkeley Lab, Japan’s Nuclear Science and Engineering Directorate and Rolls Royce.
The Office of Naval Research helped support this work. Experiments were performed at the National Center for Electron Microscopy in the Molecular Foundry at Berkeley Lab, which is supported by the U.S. Department of Energy.


Surely there must be some alloy that can be as light but as effective as titanium but is not prone to be damaged by water.