Ask an organic chemist what the meaning of life is, and he or she will probably tell you it’s the carbon-carbon bond.
Just about every biological molecule — the proteins and sugars that make up our cells, that make up us — is built on vast networks of these ubiquitous chemical bonds.
And almost every significant carbon-based biomolecule contains a nitrogen compound, or amine.
Achieving this carbon-nitrogen bond in the lab, though, is tricky business. Drug companies know it well; when synthesizing new chemicals for drug testing, they must first create the carbon-carbon bonds, and then introduce the nitrogen to make a molecule that will do something useful. It’s a multi-step, expensive and complex process.
Colorado State University chemists have figured out how to do it in one step.
One reaction for precision chemistry
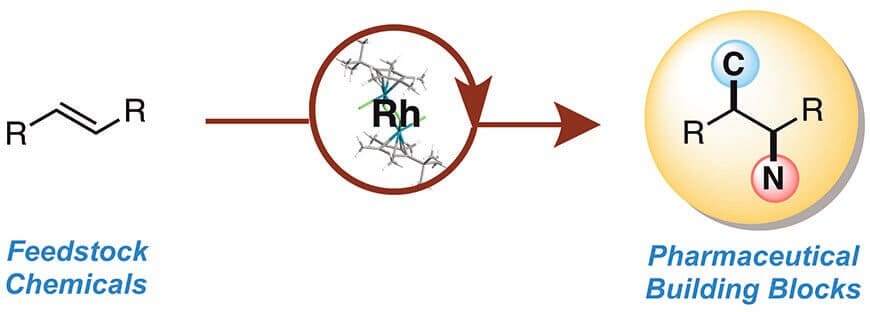
They’ve developed a process that, in the world of catalytic chemistry, achieves something remarkable: A single chemical reaction that couples two constituent chemicals into a carbon-carbon bond, while simultaneously introducing a nitrogen component. They can control the reaction to make the nitrogen atoms go exactly where they want them to, making for precision chemistry that could revolutionize pharmaceutical and materials manufacturing.
The unprecedented achievement is detailed in the journal Nature, published Oct. 21. The work was led by organic chemist Tomislav Rovis, professor of chemistry in the College of Natural Sciences at CSU, and postdoctoral researcher Tiffany Piou, who designed all the chemical building blocks and ran the experiments.
Their starting materials are oil refinery byproducts called olefins, or alkenes. They mixed in a specially engineered reagant, then used a complex based on the precious metal rhodium to reliably and specifically trigger the elusive carbon-nitrogen bonds.
Selecting single isomers
But the innovation of this new carboamination tool doesn’t stop there. Chemistry happens in three dimensions, and molecules often assemble in different shapes, or isomers. Some of these isomers are mirror images, like right and left gloves, and though they’re chemically identical, their functionalities are strikingly different. Being able to select for a single isomer is critical to safety and efficacy – so much so that the FDA mandates that only single-isomer drugs be marketed for human use.
Take thalidomide, infamous for causing severe birth defects when taken by pregnant women in the 1950s. Chemically, thalidomide comes in two mirror-image isomeric forms. One caused the defects, one didn’t.
“For this reason, spatial display of groups in molecules is incredibly important,” Rovis said. “Tiffany’s finding gives us a leg up to do this in a carboamination reaction, by making the carbon carbon bond, and delivering the nitrogen selectively.”
The researchers hope their approach, which they liken to a tool in a toolbox, can be polished, perfected and used widely to make organic chemistry easier, and applied to many different fields.