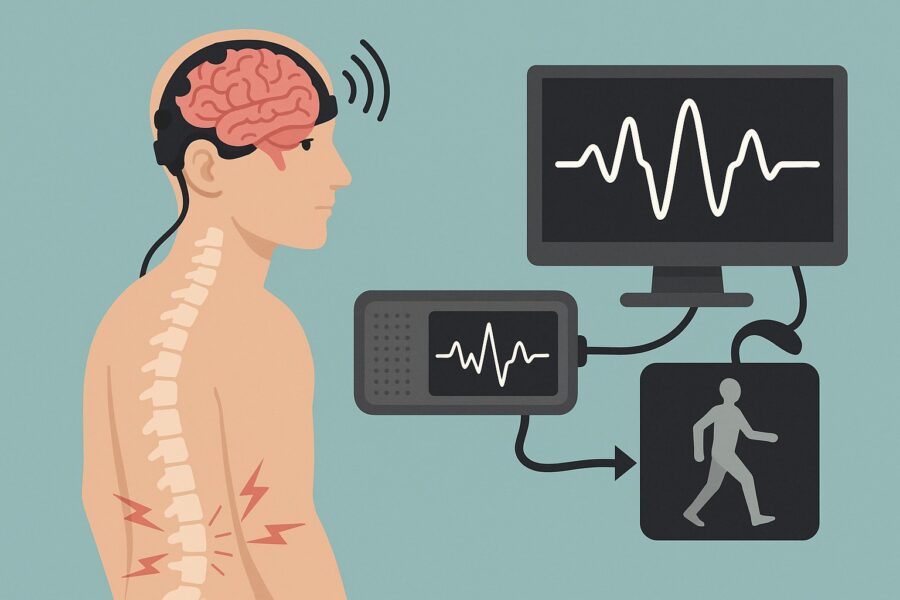
In a significant development for spinal cord injury treatment, researchers at Washington University in St. Louis have created a neural decoder that bridges the communication gap between brain and spine, potentially opening new avenues for rehabilitation.
The research team, led by Ismael Seáñez, assistant professor of biomedical engineering at WashU, demonstrated that brain activity can be interpreted and used to deliver precisely timed electrical stimulation to the spinal cord. Their findings, published April 25 in the Journal of NeuroEngineering and Rehabilitation, represent a critical step toward non-invasive rehabilitation technologies for people with paralysis.
“After we give the decoder this data, it learns to predict based on neural activity whenever there is movement or no movement,” Seáñez said. “We show that we can predict whenever someone is thinking about moving their leg, even if their leg does not actually move.”
When someone suffers a spinal cord injury, the normal communication pathway between brain and muscles becomes interrupted, resulting in paralysis despite both the brain and lower spinal circuits remaining functional. This new approach aims to reconnect these systems using external technology.
The study enlisted 17 able-bodied participants who wore specialized EEG (electroencephalography) caps fitted with electrodes to measure brain activity. Participants were asked to perform knee extensions while seated, and also to merely imagine the same movement without actually moving. This data allowed the researchers to train an algorithm to recognize the brain’s movement intentions.
What makes this approach particularly promising is its entirely non-invasive nature. Unlike other brain-spine interfaces that require surgical implantation of electrodes, this system uses external EEG caps and transcutaneous spinal cord stimulation – electrical pulses delivered through the skin.
During testing, the system achieved an impressive 83% accuracy in predicting movement intention from brain signals alone. Perhaps more remarkably, the decoder could successfully identify when participants were merely imagining leg movement with 77% accuracy, suggesting the system could potentially work for people with complete paralysis.
The commercial implications could be substantial. Current rehabilitation approaches for spinal cord injury typically reach a plateau after about six months. Technologies that reestablish communication between brain and spinal circuits could potentially extend therapeutic benefits beyond this conventional limitation.
For investors watching the neurotech space, this represents a potential inflection point. Brain-computer interfaces have attracted significant venture capital in recent years, mostly focused on invasive technologies requiring surgery. A non-invasive alternative that demonstrates clinical efficacy could dramatically expand market accessibility.
The technology works by detecting characteristic changes in brain wave patterns called “event-related desynchronization” that occur in specific frequency bands when someone intends to move. The system then uses these patterns to trigger spinal stimulation precisely when movement is intended.
“One, that it’s more likely that we’re decoding movement intention and not an artifact, or noise, and second, whenever we employ this on people with spinal cord injury who will not have that ability to actually move their legs for us to label the data, we could use their imagination of moving a leg to train our decoder,” Seáñez explained.
From a technical perspective, the system is relatively straightforward compared to other neural interfaces. It employs a linear discriminant analysis (LDA) algorithm – a well-established machine learning technique that offers interpretability and efficiency without requiring massive computational resources.
While still in early development, the researchers are already looking toward clinical applications. They plan to test whether a generalized decoder trained on data from multiple participants could perform as well as personalized ones, potentially simplifying deployment in clinical settings.
The market for spinal cord injury rehabilitation technologies remains underserved, with approximately 18,000 new cases occurring annually in the United States alone. Current rehabilitation costs can exceed $500,000 in the first year post-injury, creating strong economic incentives for more effective treatments.
For policymakers, this research highlights the growing potential of non-invasive neurotechnologies to address significant medical challenges without the risks and costs associated with surgical interventions. As these technologies mature, regulatory frameworks may need updating to account for brain-controlled medical devices that don’t require physical implantation.
While promising, several challenges remain before clinical deployment. The system currently works best with precisely timed, cued movements rather than natural, self-initiated ones. The researchers found significant differences in brain activity patterns between cued and uncued movements, suggesting that additional refinement will be necessary for real-world applications.
The research was funded by the McDonnell Center for Systems Neuroscience at Washington University in St. Louis, the National Institutes of Health, and departments at Washington University.
As neural interface technologies continue advancing, this non-invasive approach could represent a more accessible alternative to surgical solutions, potentially accelerating rehabilitation innovation while reducing patient risk. The question now becomes how quickly this technology can move from laboratory demonstration to clinical application – and whether it can fundamentally change recovery trajectories for those living with spinal cord injuries.
Discover more from NeuroEdge
Subscribe to get the latest posts sent to your email.

1 thought on “Brain decoder controls spinal cord stimulation”