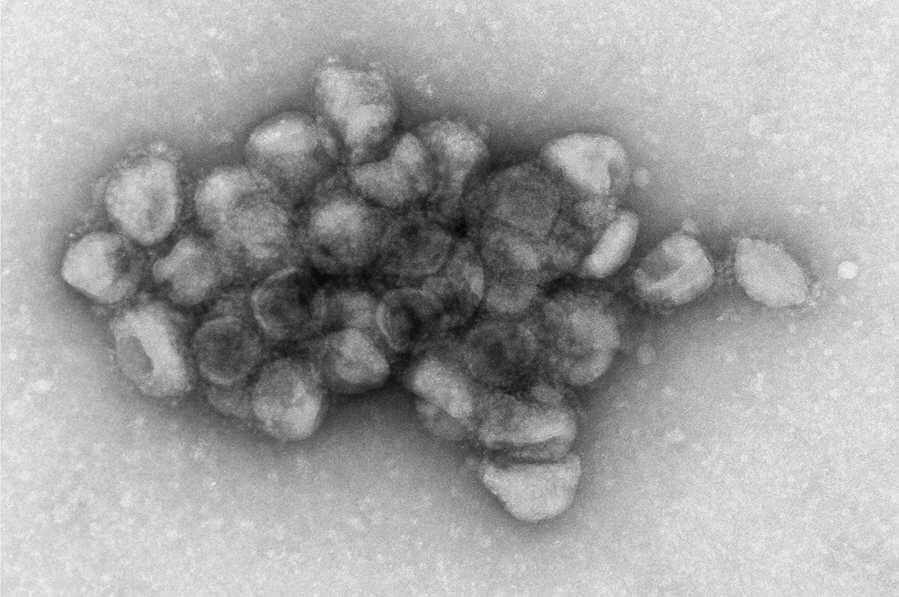
Unlike other SARS-CoV-2 variants, the Delta variant can attach to copies of itself, forming larger aggregations, or clumps, of viral particles, suggests a study by scientists at the National Institutes of Health. The researchers theorize that this linking property may have played a role in the ability of the Delta variant to spread more rapidly than all the variants that preceded it.
The study was conducted by Jennifer D. Petersen, Ph.D., of NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, and colleagues. It appears in Viruses.
The Delta spike protein enables the virus to bind to cells and begin the process of infecting them. In laboratory studies, the researchers observed this action by using leukemia viruses from mice that were stripped of disease-causing genes but engineered to have the spike protein on their surface, known as pseudotyped Delta particles. The scientists watched the spike proteins binding to one another to form aggregations, which previous research suggests increases the chances of viral spread.
Viruses deep inside the aggregation are protected from drying out, from antiviral drugs and from the host immune system. Moreover, large viral aggregations have the potential to a bring a greater number of viruses in contact with target cells, thereby increasing the chances of infection. The authors note that future studies are needed to confirm whether the SARS-CoV-2 Delta variant can form aggregations similar to those of the Delta pseudo particles.
The researchers also found that engineered particles of the Omicron variant, which has since replaced the Delta variant, do not form aggregations. However, the researchers believe that learning how Delta aggregates — and how to develop drugs that can reach viruses inside the aggregation—may prove useful if a future variant capable of aggregation arises.