If smoke indicates a fire, nitric oxide signals inflammation. The chemical mediator promotes inflammation, but researchers suspect it can do its job too well after anterior cruciate ligament (ACL) ruptures and related injuries and initiate early onset osteoarthritis. Typically, the degenerative disease is only diagnosed after progressive symptoms, but it potentially could be identified much earlier through nitric oxide monitoring, according to Huanyu “Larry” Cheng, James E. Henderson Jr. Memorial Associate Professor of Engineering Science and Mechanics at Penn State.
Cheng and his student, Shangbin Liu, who earned a master’s degree in engineering science and mechanics at Penn State this year, collaborated with researchers based in China to develop a flexible biosensor capable of continuous and wireless nitric oxide detection in rabbits. They published their approach in the Proceedings of the National Academy of Sciences.
“Real-time assessment of biomarkers associated with inflammation, such as nitric oxide in the joint cavity, could indicate pathological evolution at the initial development of osteoarthritis, providing essential information to optimize therapies following traumatic knee injury,” Cheng said.
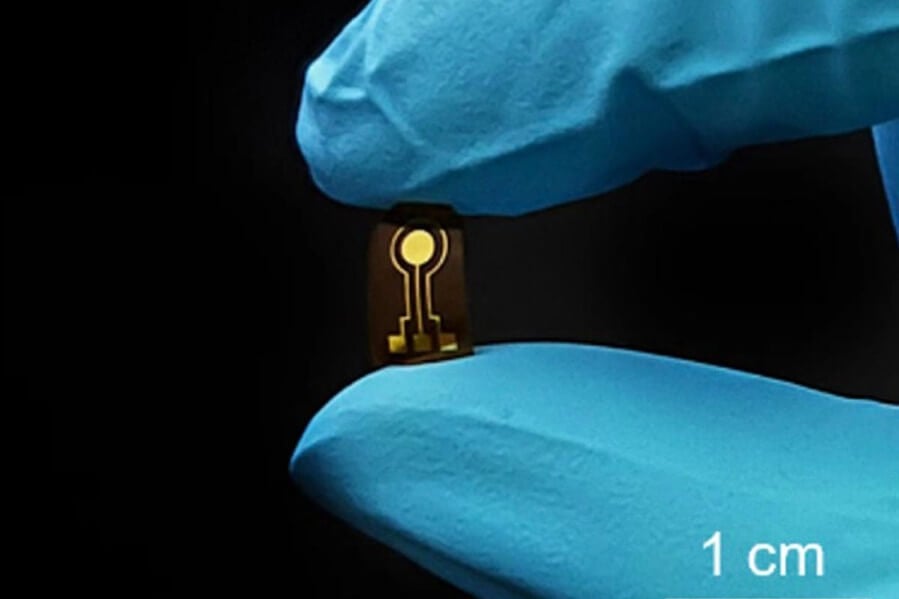
The challenge, according to Cheng, is that nitric oxide detection requires highly sensitive and stable electrochemical sensors that are also flexible and biocompatible so the geographic origins of the nitric oxide can be mapped precisely. Corresponding author Lan Yin, associate professor in the School of Materials Science and Engineering at Tsinghua University in China, previously led the development of a flexible, nitric oxide-sensitive electrochemical sensor, but it relied on an electrode configuration that restricted its capabilities.
“The limited surface area made it challenging to simultaneously attain both high sensitivity and high spatial resolution,” Yin said, meaning the device may be able to detect nitric oxide, but may not be able to pinpoint exactly where it originated, so it was not clear if the signal was related to the injury site or surrounding tissue. “Recalibration was also required on a regular basis to ensure desirable accuracy.”
The researchers turned to the potential solution of flexible and biocompatible organic electrochemical transistors (OECTs), which can use voltage and currents to identify and amplify signals. Even small ion concentrations are detectable and amplifiable once they oxidize on the gate electrode and drive ions of the electrolyte into the device’s channel; however, the channel is made of a polymer, known as PEDOT:PSS, that often operates at a different gate voltage than nitric oxide.
“We tuned the channel geometry and gate materials to align how the nitric oxide electrochemical signals enter the channel and how the device detects them, optimizing the sensing capabilities,” Cheng said. “The reference-free sensor with a miniaturized active sensing region enables nitric oxide detection with improved spatial resolution compared with previously reported electrochemical nitric oxide sensors, which could allow the mapping of electrochemical signals to offer comprehensive diagnostic information.”
The researchers integrated the sensors with a customized circuit module, resulting in a device that continuously and wirelessly monitors nitric oxide levels, which are transmitted via Bluetooth to a cell phone app. To test the design, the researchers implanted the devices in rabbits. Over eight days, the researchers found that the devices successfully detected nitric oxide concentrations.
“The results indicate that early signs of high nitric oxide concentrations could be correlated with inflammation and cartilage degeneration at the later stage, which could potentially offer essential information to evaluate the progression to osteoarthritis after ACL injury and optimize posttraumatic treatments,” Cheng said.
The researchers plan to continue investigating the connection between nitric oxide concentrations and osteoarthritis and refining the sensing technology, according to Cheng.
“Overall, the proposed materials options and device design could offer critical engineering basis for decoding health conditions at an early stage and maximizing therapeutic outcomes of associated degeneration and disorders,” Yin said.
Other contributors include Yuping Deng, Zhenhu Guo, Kuntao Chen and Lingyun Zhao, Tsinghua University; Hui Qi, Yongsheng Jie, Rui Zheng and Jinzhu Jing, Beijing Institute of Traumatology and Orthopaedics; Yuan Ma, Milin Zhang, Kaiyuan Zhang and Xing Sheng, Tsinghua University; Mingyou Zhao, Peking University; He Ding and Guoqing Lv, Beijing Institute of Technology; and Rongfeng Li, Beijing Institute of Collaborative Innovation. Guo is also affiliated with Central South University.
The National Natural Science Foundation of China, the Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program, the Beijing Municipal Health Commission and the University of Tokyo-Tsinghua Collaborative Research Fund supported this research.