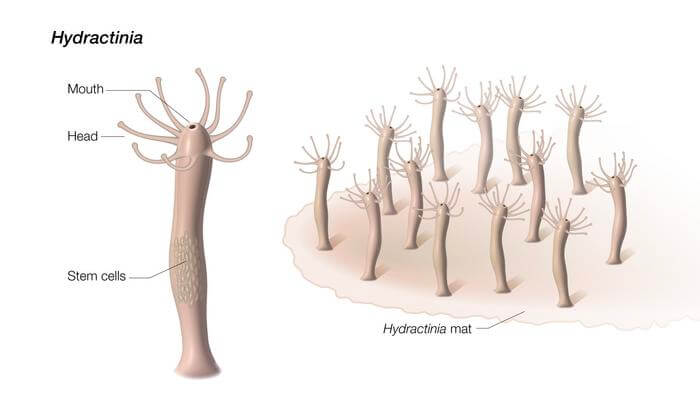
Picture this: A mesmerizing underwater world where a tiny, tube-shaped sea creature performs its sorcerous trick of regenerating an entire body from its mouth.
This creature, as alien as it may seem, holds clues to unraveling the enigmas of human aging and healing. Yes, you heard it right. Researchers at the National Institutes of Health (NIH) and their creative cohorts dived into the molecular labyrinth of Hydractinia symbiolongicarpus, a minuscule marine marvel that makes a home on hermit crab shells.
As these captivating Hydractinias kick-started their body regeneration process, the scientists deciphered a peculiar molecular signature. This molecular cryptograph was none other than senescence, a term usually whispered in the halls where biologists discuss aging.
Hydractinia’s performance under the microscope opened the doors to an intriguing thesis: the roots of healing and aging are entangled within the double helix of life, offering a fresh lens to gaze into the mysterious evolution of aging.
Charles Rotimi, Ph.D., the maestro orchestrating the Intramural Research Program at the National Human Genome Research Institute (NHGRI), part of NIH, elucidates, “Studies like this that explore the biology of unusual organisms reveal both how universal many biological processes are and how much we have yet to understand about their functions, relationships and evolution.” He added, with an air of anticipation, “Such findings have great potential for providing novel insights into human biology.”
Welcome to a new era where decoding the ancestry of biological processes like aging and healing is not only cool but quintessential to comprehend the mystique of human health and afflictions. Humans have a dash of regenerative prowess – mending broken bones or even pulling a Phoenix by regrowing a damaged liver. But then you have the likes of salamanders and zebrafish, who with their biological bravado, replace entire limbs and rejuvenate an assortment of organs. And yet, it’s the seemingly simplistic organisms like Hydractinia that wield the most formidable regenerative powers, essentially playing God by growing whole new bodies from mere tissue fragments.
Here’s where things get juicier. Senescence, in the case of Hydractinia, takes on the role of the regenerative virtuoso, which is in stark contrast to its rather gloomy reputation in human cells. Andy Baxevanis, Ph.D., the NIH’s senior science maestro, takes the stage, stating, “Most studies on senescence are related to chronic inflammation, cancer and age-related diseases.” He adds, “Typically, in humans, senescent cells stay senescent, and these cells cause chronic inflammation and induce aging in adjacent cells. From animals like Hydractinia, we can learn about how senescence can be beneficial and expand our understanding of aging and healing.”
The plot thickens as we learn that Hydractinia has its very own regenerative dream team – an elite group of stem cells. Humans have stem cells, but they mostly chill during development. Hydractinia’s stem cells, on the other hand, have no chill; they hustle throughout its lifetime. These stem cells, usually parked in the lower trunk, somehow jump into action even when the mouth – which is in a totally different neighborhood – is removed and regenerates an entire body.
It’s like the cells have secret handshakes that turn them into stem cells in times of crisis. The researchers theorized that Hydractinia must be generating new stem cells and, like detectives on the trail, searched for the molecular signals directing this spectacle.
The spotlight turned to senescence as RNA sequencing pointed its finger. The researchers played detective and looked for genome sequences resembling human
senescence genes. One of the three genes they identified came alive in cells near the amputation site. Deleting this gene meant senescent cells were blocked from joining the party, and no new stem cells or regeneration took place.
What’s next is nothing short of spellbinding. The researchers tracked these senescent cells in Hydractinia to see how they manage the trick of turning senescence into a force of good. Astonishingly, the creatures expelled the senescent cells through their mouths, a luxury humans can’t afford.
Our kinship with Hydractinia and its brethren – jellyfish and corals – stretches back over 600 million years. These beings seemingly defy time, as they don’t age. The secrets they hold might whisper tales of our earliest ancestors and suggest that regeneration could have been senescence’s original gig.
Dr. Baxevanis chimes in, “We still don’t understand how senescent cells trigger regeneration or how widespread this process is in the animal kingdom.” With a hopeful sparkle in his eyes, he continues, “Fortunately, by studying some of our most distant animal relatives, we can start to unravel some of the secrets of regeneration and aging — secrets that may ultimately advance the field of regenerative medicine and the study of age-related diseases as well.”
So, as we set our sights on the seemingly infinite depths of the biological cosmos, Hydractinia reminds us that answers to age-old questions might just be lurking in the ethereal ballet of a tiny sea creature regenerating its body from its mouth. The marine maestro bows, the curtain falls, but the exploration continues.