Targeted Therapy Reduces Harmful Protein Buildup in Brain
Researchers from Tokyo Medical and Dental University (TMDU) have developed a new approach to treating Parkinson’s disease that could potentially slow or halt its progression. The study, published in Acta Neuropathologica Communications, demonstrates how a type of genetic therapy called antisense oligonucleotides (ASOs) can reduce the buildup of harmful protein aggregates in the brain.
Parkinson’s disease, which affects millions worldwide, is characterized by the abnormal accumulation of a protein called α-synuclein (aSyn) in brain cells. These protein clumps, known as Lewy bodies, spread throughout the brain and contribute to the death of neurons, leading to the disease’s debilitating symptoms.
A Novel Approach to Tackling Protein Aggregates
The research team, led by Professor Tetsuya Nagata, explored the use of ASOs to reduce the expression of aSyn in a mouse model of Parkinson’s disease. ASOs are short pieces of DNA or RNA that can be designed to target specific messenger RNA molecules, effectively decreasing the production of corresponding proteins.
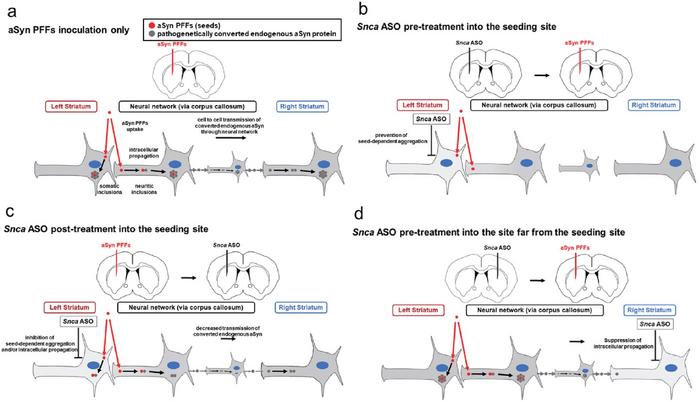
“Currently, antibody drugs and vaccines targeting aSyn are under development for aSyn-related neuropathies, but their effects may not prevent the disease from progressing inside cells,” explains Professor Nagata. “In contrast, nucleic acid drugs like ASOs that specifically control intracellular levels of normal aSyn, while also suppressing pathogenic aSyn propagation, could offer higher safety and efficacy by both retaining the natural physiological functions of the protein while inhibiting the spread of pathogenic aSyn.”
The researchers injected ASOs directly into specific regions of mouse brains and analyzed the spread of aSyn pathologies. When ASOs were administered two weeks before introducing disease-causing fibrils, they observed a significant decrease of over 90% in Lewy body-like structures. Even when ASOs were given at the same time or after the introduction of fibrillar aSyn, there was a notable inhibitory effect on the spread of aggregates.
Interestingly, the team found that administering ASOs at locations distant from the initial disease site also provided protection to other brain regions. This suggests that the treatment could potentially be effective at various stages of the disease and in different parts of the brain.
Professor Takanori Yokota, another author of the study, highlights the significance of their findings: “For the first time, we demonstrated in vivo that suppressing aSyn expression can inhibit the progression of pathology from Lewy neurites to Lewy bodies, with some of these effects being reversible. We also observed the inhibitory effect of ASO on pathology progression in regions distant from the injection site of fibrous aSyn. This suggests the potential for treating PD, dementia with Lewy bodies, multiple system atrophy, and other aSyn-based diseases at various stages by reducing endogenous aSyn via ASOs without completely depleting this vital protein.”
Why it matters: This research presents a promising new avenue for treating Parkinson’s disease and other neurodegenerative disorders characterized by protein aggregation. Current treatments for Parkinson’s primarily focus on managing symptoms rather than addressing the underlying cause of the disease. If successful in human trials, this ASO-based approach could potentially slow or halt disease progression, dramatically improving the quality of life for millions of patients worldwide.
The study’s findings are particularly exciting because they suggest that ASO treatment could be effective at different stages of the disease and in various brain regions. This versatility could make it a valuable tool in the fight against neurodegenerative disorders, which often progress in complex and unpredictable ways.
However, it’s important to note that this research is still in its early stages. While the results in mice are encouraging, human trials will be necessary to determine the safety and efficacy of this approach in people. Questions remain about the long-term effects of reducing aSyn levels in the brain, as this protein plays important roles in normal neuronal function.
Looking ahead, this research opens up new possibilities for targeted therapies in neurodegenerative diseases. Future studies may explore optimizing ASO delivery methods, investigating potential side effects, and examining the treatment’s efficacy in more advanced stages of the disease. As our understanding of the molecular mechanisms underlying these disorders grows, so too does the potential for developing more effective and personalized treatments.