Researchers have discovered a link between prostate cancer aggressiveness and the accumulation of a compound produced when cholesterol is metabolized in cells, findings that could bring new diagnostic and treatment methods.
Findings also suggest that a class of drugs previously developed to treat atherosclerosis might be repurposed for treatment of advanced prostate cancer.
The research showed depletion of the compound cholesteryl ester significantly reduced prostate cancer cell proliferation, impaired its ability to invade a laboratory tissue culture and suppressed tumor growth in mice.
“Our study provides an avenue towards diagnosis of aggressive prostate cancer. Moreover, we showed that depleting cholesteryl ester significantly impairs prostate cancer aggressiveness,” said Ji-Xin Cheng, a professor in Purdue University’s Weldon School of Biomedical Engineering and Department of Chemistry.
The research involved analysis of clinical samples harvested from prostate cancer patients, specialized cell lines and mice.
Findings are detailed in a research paper appearing Tuesday (March 4) in the journal Cell Metabolism. The paper was authored by researchers associated with Purdue’s Center for Cancer Research and the Indiana University Melvin and Bren Simon Cancer Center at Indiana University School of Medicine.
“Prostate cancer is the second-leading cause of cancer-related mortality in American men. Our finding offers a biological foundation that supports the beneficial effect of cholesterol-lowering drugs. Second, our study heralds the potential of using cholesteryl ester as a therapeutic target for advanced prostate cancer,” said study co-author Timothy Ratliff, the Robert Wallace Miller Director of Purdue’s Center for Cancer Research. “These results together suggest that cholesteryl ester accumulation might be used for more accurate prediction of prostate cancer aggressiveness, if validated through further examination of a large number of tissue biopsies and correlation assessment of cholesteryl ester levels and clinical outcomes of patients.”
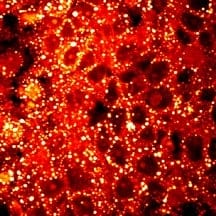
A critical focus of the research is the analysis of individual lipid droplets inside single cells. Purdue researchers have developed an analytical tool called Raman spectromicroscopy that allows compositional analysis of single lipid droplets in living cells and mice.
“It is conceivable that cancer cells require reservoirs for lipids, namely lipid droplets. However, our imaging data revealed an unexpected, aberrant accumulation of esterified cholesterol in lipid droplets of high-grade prostate cancer and metastases,” Cheng said.
The researchers learned that cholesteryl ester accumulation, which occurs only in advanced prostate cancer and its metastasis, results from the loss of a tumor-suppressing gene called PTEN and the activation of an intracellular metabolic pathway promoting tumor growth.
“These findings improve current understanding of the role of cholesterol in cancer and also suggest new opportunities for the diagnosis and treatment of aggressive prostate cancer. We have been pleased to be able to collaborate with Dr. Cheng on his important research” said Michael Koch, John P. Donohue Professor of Urology and chair of the Department of Urology at IU School of Medicine.
Findings show the drugs avasimibe and Sandoz 58-035 reduced the accumulation of cholesteryl ester and significantly hindered advanced prostate cancer growth in laboratory cell cultures and xenograft mouse models. These drugs did not show toxicity to animals.
“We note that avasimibe, Sandoz 58-035 and a class of similar drugs were developed to treat atherosclerosis, but the clinical trials were halted due to the lack of effectiveness in reducing plaque size,” Cheng said. “The present study highlights a novel use of these drugs to treat advanced prostate cancer.”
The paper was authored by Purdue doctoral students Shuhua Yue, Junjie Li, Seung-Young Lee and Hyeon Jeong Lee; Purdue postdoctoral research assistants Tian Shao and Bing Song; Liang Cheng, professor of pathology and laboratory medicine and urology at the IU School of Medicine; Timothy A. Masterson, an assistant professor of urology at the IU School Medicine; Xiaoqi Liu, an associate professor in Purdue’s Department of Biochemistry; Ratliff, a professor of comparative pathobiology; and Cheng.
The research was supported by the Walther Cancer Foundation, National Institutes of Health, National Science Foundation and the Department of Defense.