Alzheimer’s disease, the primary cause of dementia in the elderly, imposes a tremendous social and economic burden on modern society. In Japan, the burden of the disease in 2050 is estimated to be a half a trillion US dollars, a figure equivalent to the government’s annual revenues.
Unfortunately, it has proven very difficult to develop drugs capable of ameliorating the disease. After a tremendous burst of progress in the 1990s, the pace of discoveries has slowed. Dr. Saido believes that part of the difficulty is the inadequacy of current mouse models to replicate the real conditions of Alzheimer’s disease and allow an understanding of the underlying mechanisms that lead to neurodegeneration. In fact, much of the research in Alzheimer’s disease over the past decade may be flawed, as it was based on unrealistic models.
The problem with older mouse models is that they overexpress a protein called amyloid precursor protein, or APP, which gives rise to the amyloid-beta (Abeta) peptides that accumulate in the brain, eventually leading to the neurodegeneration that characterizes Alzheimer’s disease. However, in mice the overexpression of APP gives rise to effects which are not seen in human Alzheimer’s disease.
For example, the APP mutant mice often die of unknown causes at a young age, and the group believes this may be related to the generation of toxic fragments of APP, such as CTF-beta. In addition, some of the fragments of APP could be neuroprotective, making it difficult to judge whether drugs are being effective due to their effect on Abeta peptides, which are known to be involved in human AD, or whether it is due to other effects that would not be seen in human disease. In addition, the gene for expressing APP is inserted in different places in the genome, and may knock out other genes, creating artifacts that are not seen in humans.
With this awareness, more than a decade ago Dr. Saido launched a project to develop a new mouse model that would allow more accurate evaluation of therapies for the disease. One of the major hurdles involved a part of the gene, intron 16, which they discovered was necessary for creating more specific models.
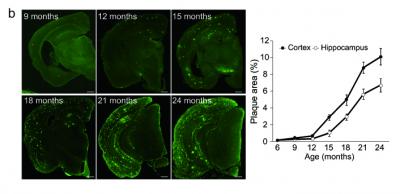
The first mice model they developed (NL-F/NL-F) was knocked in with two mutations found in human familial Alzheimer’s disease. The mice showed early accumulation of Abeta peptides, and importantly, were found to undergo cognitive dysfunction similar to the progression of AD seen in human patients. A second model, with the addition of a further mutation that had been discovered in a family in Sweden, showed even faster initiation of memory loss.
These new models could help in two major areas. The first model, which expresses high levels of the Abeta peptides, seems to realistically model the human form of AD, and could be used for elucidating the mechanism of Abeta deposition. The second model, which demonstrates AD pathology very early on, could be used to examine factors downstream of Abeta-40 and Abeta-42 deposition, such as tauopathy, which are believed to be involved in the neurodegeneration. These results may eventually contribute to drug development and to the discovery of new biomarkers for Alzheimer’s disease. The group is currently looking at several proteins, using the new models, which have potential to be biomarkers.
According to Dr. Saido, “We have a social responsibility to make Alzheimer’s disease preventable and curable. The generation of appropriate mouse models will be a major breakthrough for understanding the mechanism of the disease, which will lead to the establishment of presymptomatic diagnosis, prevention and treatment of the disease.”


Revolting… Skanky… Yucky… Disgusting… This is the thoughts that come to mind when the average man on the street hears the word ‘mice’. In contrast, mice can be seen as one of the most precious assets the scientific community, and by extrapolation, ordinary everyday people, will ever lay hands on. Think about it – where would we be if it wasn’t for those modest little mice that were bred with the pure purpose of fulfilling the destiny of man – to become as immortal as possible. From research in finding cures for incurable diseases and medication to treat them, to developing beauteous make-up to please the confidence of the fairer sex and the eyes of the men who gasp at their fairness, mice are at the forefront of our advancement. Face it – without that ‘nasty little vermin’ we might as well have still been living in the Stone Age. I think we should show a bit more appreciation towards mice the next time the topic surfaces in conversation.
I had posted the comment before on this issue , this very detailed reference below regarding `Viruses as Modulators of Mitochondrial function` – this simple clue(mitochondrial viruses) may help the studies at the cellular organelle level – or at least direct the basic researchers towards the right solution – to find a cure or at least effective treatment and prevention for the Alzheimer’s Disease .
Thanks
http://www.hindawi.com/journals/av/2013/738794/ Viruses as Modulators of Mitochondrial Functions
Can Alzheimer’s disease be caused by the human body being overloaded with unknown compounds and proteins? I believe mice can not truly help with this problem. Some individuals react differently to a certain disease than others ad even more so their reactions differ from those of animals. I do not say that we should be testing on humans, but rather on living cells. The problem with it all is the lack of understanding on how the disease functions. This problem can only be solved by a study of the human-cells not animal-cells.
My personal opinion in this subject is : Alzheimer`s disease is the disease and permanent irreplaceable death of high energy demanding Neuron cells of the Brain –like memory function neurons- due to `lack of adequate energy from mitochondria’s` – hundreds of energy producing intra cytoplasmic organelles with their own identical genes called m DNA of Nerve cells – Alzheimer`s disease is simply the END RESULT OF `ABSENCE OR INADEQUACY OF ENERGY PRODUCTION BY THE MITOCHONDRIAS WITHIN THE NEURONS AND ULTIMATELY DIRECTLY LACK OF ENERGY RELATED DEATH OF NEURONS OF THE BRAIN .
All other molecular changes and accumulation of chemicals (proteins) are related to `secondary events and innocent bystanders` after the Neuronal death but not the primary cause rather than after the fact of neuronal death .
So ; the helpful clue for the proper use of mouse models for Alzheimer`s research and potential treatment modalities is : Try to modify the mouse `MITOCHONDRIAL` gene and design to evaluate the nuclear chromosomal gene mutual interactions and molecular level communication in between Mitochondria and the nucleus of the Nerve cells based research should lead to the `fundamental` understanding at the molecular level ; the etiology and the ultimate treatment of Alzheimer`s disease .
Unless Mouse Nerve cells `mitochondrias` are manipulated and altered experimentally in genetic terms (m DNAs) , mouse models can not give any clue what is `Alzheimer`s disease all about and how to treat or prevent it .
Helpful summary and recent research on overall compilation of association of Mitochondrial dysfunction with Alzheimer’s at the link below :
Full-Text PDF – Scientific Research Publishing Mitochondrial Dysfunction and Alzheimer’s Disease