Not too long ago the idea of taking, say a skin cell, and transforming it into a muscle cell was unthinkable. About 10 years ago, however, revolutionary research showed that it is indeed possible to reprogram differentiated adult cells into other types fully capable of conducting new functions.
Cell reprogramming is a main interest of the lab of Dr. Todd Rosengart, chair and professor of the Michael E. DeBakey Department of Surgery at Baylor College of Medicine, whose research focuses on finding innovative therapeutic approaches for heart failure.
After a heart attack, the parts of the heart muscle that die do not regenerate into new heart tissue; instead, they are replaced by a scar that does not help the heart to beat. “The idea behind cell reprograming is to coach the heart to heal itself by inducing the scar tissue, which is made mostly of fibroblasts, to change into functional heart muscle,” said Rosengart, professor of heart and vascular disease at the Texas Heart Institute.“Heart failure remains the leading cause of death from heart disease,” said Rosengart, DeBakey-Bard Chair in Surgery and professor of molecular and cellular biology at Baylor. “Nearly 5 million Americans can be expected to develop advanced congestive heart failure, and heart transplant or mechanical circulatory support implantation currently are the only options for patients with end-stage heart disease. However, these options are limited. We need to improve how to treat this devastating condition.”
Researchers have succeeded at reprograming fibroblasts from small animals to become heart muscle, with dramatic improvements in heart function.
In this study, Rosengart and his colleagues explored a novel strategy to enhance the reprogramming efficiency of human fibroblasts.
“While human fibroblasts resist being reprogramed, endothelial cells, those that line the blood vessels, are known to be more flexible – they have the capacity to naturally transdifferentiate or change into other cells,” said co-first author Dr. Megumi Mathison, associate professor of surgery at Baylor. “This gave us the idea of using this endothelial cell plasticity to improve the reprograming efficiency.”
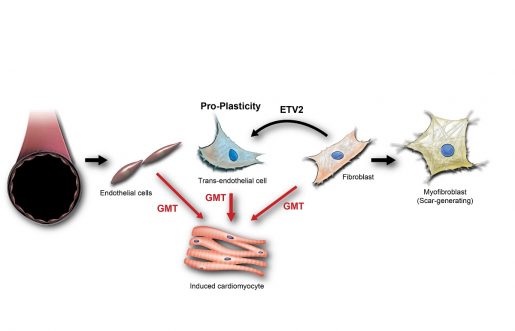
The researchers’ idea was to first induce fibroblasts to transition into an endothelial cell-like state and then treat these cells with their reprograming cocktail that directs them to change into cardiomyocytes. The expectation was that transitioning into endothelial cell-like cells, a cell type more open to reprogramming than fibroblasts, would facilitate the desired change into heart muscle.
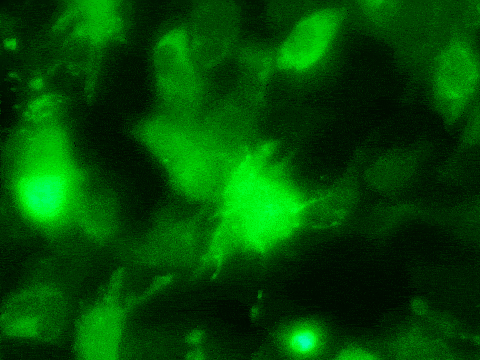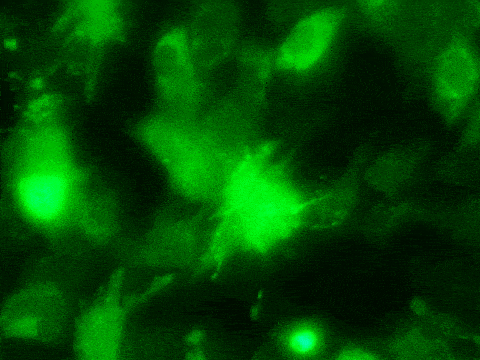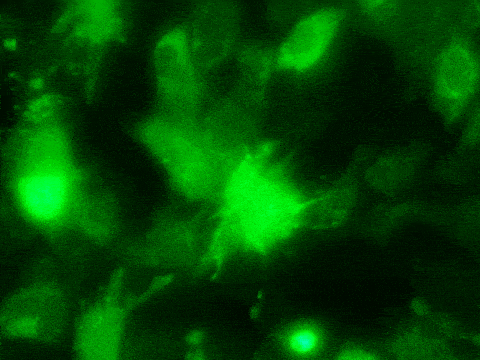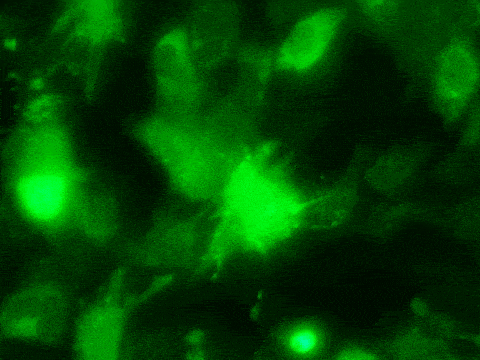
“We were delighted to see that our approach significantly enhanced reprogramming efficiency both in human and rat fibroblasts,” Mathison said. “Previously, inducing cardiomyocytes from fibroblasts directly was only 3% efficient. With our new approach, the efficiency increased 5 times. It took about two to three weeks for the fibroblasts to transition into cardiomyocytes in the lab. It was exciting to see the reprogrammed cells contracting in synchrony with surrounding cardiomyocytes.”
“Although more research is needed, we anticipate that this novel approach can become part of the next generation of biological therapies,” Rosengart said. “In a future scenario, patients with congestive heart failure would come to the catheterization laboratory, commonly referred to as the cath lab, in a hospital. The cath lab has diagnostic imaging equipment that helps surgeons visualize the chambers of the heart and surrounding blood vessels as they conduct procedures. Assisted by this equipment, the surgeon would inject the factors that promote the transition from fibroblasts to endothelial cells and then to cardiomyocytes directly into the heart. Follow ups would monitor the progress of the procedure.”
This work strongly ties into the prominent role Baylor College of Medicine has played in the history of the artificial heart and heart transplants pioneered by Dr. Michael E. DeBakey and Dr. Denton A. Cooley in the 1950s and 60s.
“Years ago, Dr. Cooley said to me, regarding the next procedures needed to help people with heart failure, ‘Todd, you got to do something that is dramatic.’ For me, cell reprograming is a 21st century answer to his request,” Rosengart said.
Find all the details of this work in the journal Scientific Reports.
Other contributors to this work include Deepthi Sanagasetti, Vivek P. Singh, Aarthi Pugazenthi, Jaya Pratap Pinnamaneni, Christopher T. Ryan and Jianchang Yang, all at Baylor College of Medicine.
This study was funded by the National Heart, Lung and Blood Institute (R01HL121294‐01A1, R01HL 152280, 5T32HL139430), the Baylor College of Medicine Cytometry and Cell Sorting Core (National Institutes of Health grants P30AI036211, P30CA125123, S10RR024574; the National Institute of Allergy and Infectious Diseases grant AI036211), and the Baylor College of Medicine Integral Microscopy Core (NIH DK56338, CPRIT RP150578, and RP170719).