Study shows how changes in the organized cell membrane network of heart muscle leads to heart failure
Colchicine, a drug that’s used to treat gout, has the beneficial side effect of lowering the risk of heart attack in patients taking it. Conversely, taxol, a drug for treating cancer, has the opposite effect; raising the risk of heart failure.
What both these drugs have in common is that they act on microtubules – a network of fibers inside heart cells that provide internal structural support. Previous studies, including evidence from human patients and experimental models of heart failure, have suggested a link between heart failure and increased density of microtubules, but the mechanisms underlying the link were not clear.
“While it was known that changes in microtubules are linked to the progression of heart failure, no one understood how this was happening,” says Dr. Long-Sheng Song,associate professor of internal medicine at the University of Iowa Carver College of Medicine. “Our study provides the first compelling evidence showing how increased microtubule density drives heart failure.”
The new study, published online Feb. 11 in the journal Circulation, reveals that colchicine, which decreases the density of microtubules, protects normal heart muscle function and increases survival in mice with heart failure, while taxol, which increases microtubule density, accelerates heart muscle damage during heart failure.
Specifically, the UI team, led by Song and first study author Caimei Zhang found that increasing microtubule density causes a specialized protein called junctophilin-2 (JP2) to be shuttled to the wrong cellular destination. This abnormal redistribution of JP2 leads to loss of normal heart cell function and ultimately heart failure.
“Changes in microtubule density directly affect where junctophilin-2 is located within the cell,” Song explains. “If junctophilin-2 is not in its proper destination, then the cell cannot function properly, which leads to the development and progression of heart failure.”
Location, location, location
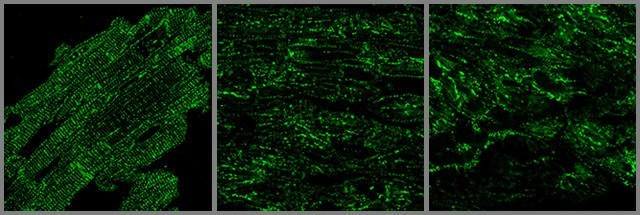
JP2 protein is critical to the normal organization of an important component of heart muscle membrane network called Transverse-tubules or T-tubules. These T-tubules are essential for transmitting the electrical and chemical signals that make a heart beat.
The UI study shows that increased density of microtubules results in abnormal localization of JP2 away from the T-tubule sites to the periphery of the heart cell. The researchers show that this abnormal distribution pattern is also seen in animal models of heart failure and in failing human heart muscle.
Using a mouse model of heart failure, the UI researchers found that decreasing microtubule density with the gout drug colchicine prevents the abnormal redistribution of JP2, prevents disruption of T-tubules, and protects the mice from heart failure.
“The findings suggest that colchicine could be considered as a treatment for patients with heart failure,” says Song. “The study also suggests that future approaches targeting junctophilin-2 directly might be a potential strategy for treating heart failure.”
In addition to Song and Zhang, the research team included UI researchers Biyi Chen; Ang Guo; Yanqi Zhu; Shan Gao; William Kutschke; Robert M. Weiss; Frances L. Johnson; and Mark E. Anderson. Researchers from the Department of Veterans Affairs Medical Center, Iowa City, Iowa; Mayo Clinic, Rochester, Minn.; Anhui Medical University in China; University of Washington School of Medicine, Seattle, Wash.; and Baylor College of Medicine, Houston, Texas, also were part of the team.
The research was supported by grants from the National Institutes of Health (HL090905, HL092235, HL079031, HL622494, HL70250, HL113001, HL085686), and the American Heart Association.