The biological information that makes us unique is encoded in our DNA. DNA damage is a natural biological occurrence that happens every time cells divide and multiply. External factors such as overexposure to sunlight can also damage DNA.
Understanding how the human body recognizes damaged DNA and initiates repair fascinates Michael Feig, professor of biochemistry and molecular biology at Michigan State University. Feig studies the proteins MutS and MSH2-MSH6, which recognize defective DNA and initiate DNA repair. Natural DNA repair occurs when proteins like MutS (the primary protein responsible for recognizing a variety of DNA mismatches) scan the DNA, identify a defect, and recruit other enzymes to carry out the actual repair.
“The key here is to understand how these defects are recognized,” Feig explained. “DNA damage occurs frequently and if you couldn’t repair your DNA, then you won’t live for very long.” This is because damaged DNA, if left unrepaired, can compromise cells and lead to diseases such as cancer.
Feig, who has used national supercomputing resources since he was a graduate student in 1998, applied large-scale computer simulations to gain a detailed understanding of the cellular recognition process. Numerical simulations provide a very detailed view down to the atomistic level of how MutS and MSH2-MSH6 scan DNA and identify which DNA needs to be repaired. Because the systems are complex, the research requires large amounts of computer resources, on the order of tens of millions of CPU core hours over many years.
“We need high-level atomic resolution simulations to get insights into the answers we are searching for and we cannot run them on ordinary desktops,” Feig said. “These are expensive calculations for which we need hundreds of CPUs to work simultaneously and the Texas Advanced Computing Center (TACC) resources made that possible.”
As a user of the National Science Foundation’s Extreme Science and Engineering Discovery Environment (XSEDE), Feig tasked TACC’s Ranger and Stampede supercomputers to accelerate his research. Ranger served the national open science community for five years and was replaced by Stampede (the sixth most powerful supercomputer in the world) in January 2013.
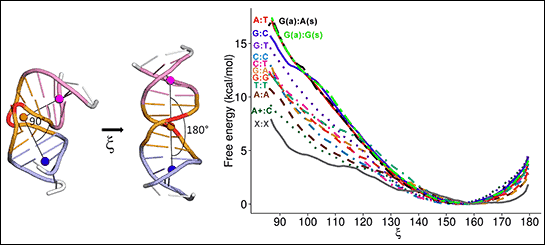
DNA chains are made of four precise chemical base pairs with distinct compositions. In a paper published in the Journal of Physical Chemistry B (April 26, 2013), Feig and his research team showed that the identification and initiation of repair depended on how the MutS protein bound with the base mismatches.
“We believe that DNA bending facilitates the initial recognition of the mismatched base for repair,” Feig said. “Normal DNA is like a stiff piece of rubber, relatively straight. It becomes possible to bend the DNA in places where there are defects.”
The biological repair machinery seems to take advantage of this propensity by ‘testing’ DNA to determine whether it can be bent easily. If that is the case, the protein has found a mismatch and repair is initiated.
“When the MutS protein is deficient in certain people, they have a high propensity to develop certain types of cancer,” Feig said. “We’re interested in understanding, first of all, how exactly this protein works. The long-term idea is to develop strategies for compensating for this protein, basically substituting some other mechanism for recognizing defective DNA and enabling repair.”
The strongest link between diseases and defects from the MutS protein has been made for a specific type of genetically inherited colon cancer.
“If an essential protein like MutS is missing or less than adequate, then the cells will not behave in a normal way,” he explained. “They will turn cancerous. The cells will refuse to die and proliferate in an uncontrollable state.”
In these cases, cancer is not a result of damaged DNA, but occurs because of a problem in the DNA repair mechanism itself.
“It probably has effects on many other cancers as well, because all the cancers are ultimately linked to defective DNA,” he said. “If DNA damage is not recognized and repaired in time then it can lead to any type of cancer. It is a fairly generic mechanism.”
According to Matt Cowperthwaite, TACC’s medical informatics programs coordinator, Feig’s research is enormously important for advancing our understanding of how cells repair the mistakes that inevitably occur during DNA replication. “For the first time, we have a mechanistic insight of how MutS finds mutations. This is extremely important research because the process of mutation underlies some of the deadliest diseases to affect humans, such as cancer.”
Research in this area, being very fundamental in nature, throws up many challenges, but its potential in future impact, Feig believes, is tremendous.
“There are many proteins with different and important biological functions,” he said. “Understanding their functions and roles in the human body will be a driving force for research in the near future.”
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!