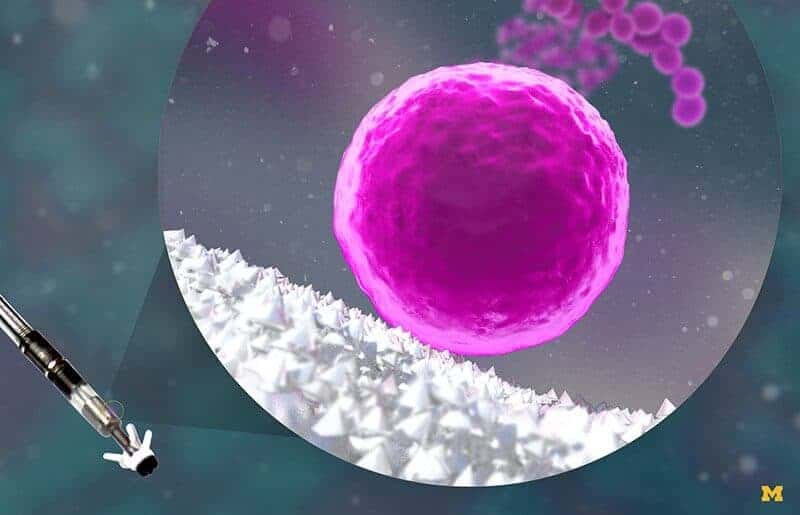
A common ingredient in sunscreen could be an effective antibacterial coating for medical implants such as joint replacements and pacemakers. Zinc oxide nanopyramids can disrupt methicillin-resistant Staphylococcus aureus (MRSA), reducing the film of bacteria growing on coated materials by over 95 percent.
About one million implanted medical devices are infected each year. “It is extremely difficult to treat these infections,” said J. Scott VanEpps, a clinical lecturer and research fellow in the department of emergency medicine, whose team led the biological study.
He explained that treatment involves a long course of antibiotics, which can lead to antibiotic resistance or toxic side-effects. “Or the implants must be surgically replaced, which can be quite extensive for devices such as heart valves and prosthetic joints,” he said.
Ideally, doctors would like to prevent the infections from occurring in the first place. One option is to coat the devices with something that bacteria can’t grow on. The new results, published in the journal Nanomedicine, suggest that such a coating could be made from zinc oxide nanoparticles.
If the nanoparticles are shaped like a pyramid with a hexagonal base, they are very effective at preventing the enzyme beta-galactosidase from breaking down lactose into the smaller sugars glucose and galactose. Human cells also employ a beta-galactosidase enzyme to break down sugars for fuel, but they are not affected until a dose of nanopyramids roughly a thousand times higher than that needed to kill bacteria.
Shape is important, both for the enzymes and the nanoparticles. The enzymes need to be able to twist in order to break down the large sugar molecules. Two amino acids, or protein building blocks, sit opposite one another across a groove in the enzyme. The lactose fits into the groove, and the amino acids come together to catalyze the breakup into glucose and galactose.
“Although more studies need to be carried out, we believe that zinc oxide nanopyramids interfere with this twisting motion,” said Nicholas Kotov, the Joseph B. and Florence V. Cejka Professor of Chemical Engineering, whose group made the nanoparticles.
The team’s research suggests that part of the nanoparticle—an edge or the point—inserts itself into the groove. By clogging up just one of the four grooves, the nanoparticles can shut down the whole enzyme by preventing the twisting action. To explore the concept of an antibacterial coating, Kotov’s group covered some pegs with the nanopyramids and then VanEpps’s team stuck them into a substance that would allow bacteria to grow. They evaluated four species of bacteria on coated and uncoated pegs—two staphylococcal species, one that causes pneumonia and E. coli.
After 24 hours of growth, the number of viable staphylococcal cells recovered from the coated pegs was 95% less than those from the uncoated pegs. The pneumonia and E. coli species were less susceptible to the nanoparticles.
“While the coating was unable to completely eradicate all staphylococcal cells, this dramatic reduction could likely enable antibiotic treatments to succeed or simply allow the human immune system to take over without the need for antibiotics,” said VanEpps.
Staph, including MRSA, is particularly vulnerable to the nanopyramids because its cell wall is a matrix of proteins and sugars. The team suspects that as the MRSA tried to colonize the pegs, the nanopyramids bound to the enzymes that build the cell wall. Since the enzymes couldn’t maintain the cell wall, the cells broke down. If this is indeed how the nanopyramids operate, then the coating should be no trouble for human cells, whose membrane enclosures don’t have the same vulnerabilities. It may also account for why the coating isn’t nearly as effective on E. coli, which doesn’t wear its cell wall enzymes on its sleeve.
Many hurdles stand between the nanoparticle coating and clinical use. The researchers must find out how such a coating would affect human cells near the implant. They must also explore how the nanopyramids affect other enzymes in humans and bacteria.
“The strong antibacterial activity against MRSA and other pathogens is an exciting finding,” said Kotov. “We want to better understand the mechanisms of the antibacterial function to fine tune its inhibitory activity and to identify the structural similarities among enzymes that pyramidal nanoparticles can inhibit.”
The paper demonstrating the effectiveness of the nanopyramid coating has just been published in Nanomedicine, titled “Zinc Oxide Nanoparticle Suspensions and Layer-By-Layer Coatings Inhibit Staphylococcal Growth.” The paper describing the antibacterial activity of pyramidal zinc oxide nanoparticles was published in ACS Nano in September, titled “Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity.”
This work was supported by the U.S. National Science Foundation, the U.S. National Institutes of Health and the National Research Foundation of Korea and the Society for Academic Emergency Medicine.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!