Caltech biologists have modified a harmless virus in such a way that it can successfully enter the adult mouse brain through the bloodstream and deliver genes to cells of the nervous system. The virus could help researchers map the intricacies of the brain and holds promise for the delivery of novel therapeutics to address diseases such as Alzheimer’s and Huntington’s. In addition, the screening approach the researchers developed to identify the virus could be used to make additional vectors capable of targeting cells in other organs.
“By figuring out a way to get genes across the blood-brain barrier, we are able to deliver them throughout the adult brain with high efficiency,” says Ben Deverman, a senior research scientist at Caltech and lead author of a paper describing the work in the February 1 online publication of the journal Nature Biotechnology.
The blood-brain barrier allows the body to keep pathogens and potentially harmful chemicals circulating in the blood from entering the brain and spinal cord. The semi-permeable blockade, composed of tightly packed cells, is crucial for maintaining a controlled environment to allow the central nervous system to function properly. However, the barrier also makes it nearly impossible for many drugs and other molecules to be delivered to the brain via the bloodstream.
To sneak genes past the blood-brain barrier, the Caltech researchers used a new variant of a small, harmless virus called an adeno-associated virus (AAV). Over the past two decades, researchers have used various AAVs as vehicles to transport specific genes into the nuclei of cells; once there, the genes can be expressed, or translated, from DNA into proteins. In some applications, the AAVs carry functional copies of genes to replace mutated forms present in individuals with genetic diseases. In other applications, they are used to deliver genes that provide instructions for generating molecules such as antibodies or fluorescent proteins that help researchers study, identify, and track certain cells.
Largely because of the blood-brain barrier problem, scientists have had only limited success delivering AAVs and their genetic cargo to the central nervous system. In general, they have relied on surgical injections, which deliver high concentrations of the virus at the injection site but little to the outlying areas. Such injections are also quite invasive. “One has to drill a hole through skull, then pierce tissue with a needle to the injection site,” explains Viviana Gradinaru (BS ’05), assistant professor of biology and biological engineering at Caltech and senior author on the paper. “The deeper the injection, the higher the risk of hemorrhage. With systemic injection, using the bloodstream, none of that damage happens, and the delivery is more uniform.”
In addition, Gradinaru notes, “many disorders are not tightly localized. Neurodegenerative disorders like Huntington’s disease affect very large brain areas. Also, many complex behaviors are mediated by distributed interacting networks. Our ability to target those networks is key in terms of our efforts to understand what those pathways are doing and how to improve them when they are not working well.”
In 2009, a group led by Brian Kaspar of Ohio State University published a paper, also in Nature Biotechnology, showing that an AAV strain called AAV9 injected into the bloodstream could make its way into the brain—but it was only efficient when used in neonatal, or infant, mice.
“The big challenge was how do we achieve the same efficiency in an adult,” says Gradinaru.
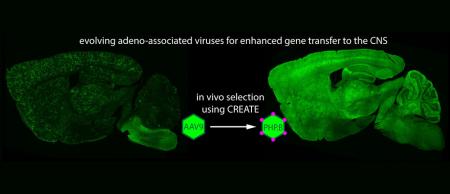
Although one might like to design an AAV that is up to the task, the number of variables that dictate the behavior of any given virus, as well as the intricacies of the brain and its barrier, make that extremely challenging. Instead, the researchers developed a high-throughput selection assay, CREATE (Cre REcombinase-based AAV Targeted Evolution), that allowed them to test millions of viruses in vivo simultaneously and to identify those that were best at entering the brain and delivering genes to a specific class of brain cells known as astrocytes.
They started with the AAV9 virus and modified a gene fragment that codes for a small loop on the surface of the capsid—the protein shell of the virus that envelops all of the virus’ genetic material. Using a common amplification technique, known as polymerase chain reaction (PCR), they created millions of viral variants. Each variant carried within it the genetic instructions to produce more capsids like itself.
Then they used their novel selection process to determine which variants most effectively delivered genes to astrocytes in the brain. Importantly, the new process relies on strategically positioning the gene encoding the capsid variants on the DNA strand between two short sequences of DNA, known as lox sites. These sites are recognized by an enzyme called Cre recombinase, which binds to them and inverts the genetic sequence between them. By injecting the modified viruses into transgenic mice that only express Cre recombinase in astrocytes, the researchers knew that any sequences flagged by the lox site inversion had successfully transferred their genetic cargo to the target cell type—here, astrocytes.
After one week, the researchers isolated DNA from brain and spinal cord tissue, and amplified the flagged sequences, thereby recovering only the variants that had entered astrocytes.
Next, they took those sequences and inserted them back into the modified viral genome to create a new library that could be injected into the same type of transgenic mice. After only two such rounds of injection and amplification, a handful of variants emerged as those that were best at crossing the blood-brain barrier and entering astrocytes.
“We went from millions of viruses to a handful of testable, potentially useful hits that we could go through systematically and see which ones emerged with desirable properties,” says Gradinaru.
Through this selection process, the researchers identified a variant dubbed AAV-PHP.B as a top performer. They gave the virus its acronym in honor of the late Caltech biologist Paul H. Patterson because Deverman began this work in Patterson’s group. “Paul had a commitment to understanding brain disorders, and he saw the value in pushing tool development,” says Gradinaru, who also worked in Patterson’s lab as an undergraduate student.
To test AAV-PHP.B, the researchers used it to deliver a gene that codes for a protein that glows green, making it easy to visualize which cells were expressing it. They injected the AAV-PHP.B or AAV9 (as a control) into different adult mice and after three weeks used the amount of green fluorescence to assess the efficacy with which the viruses entered the brain, the spinal cord, and the retina.
“We could see that AAV-PHP.B was expressed throughout the adult central nervous system with high efficiency in most cell types,” says Gradinaru. Indeed, compared to AAV9, AAV-PHP.B delivers genes to the brain and spinal cord at least 40 times more efficiently.
“What provides most of AAV-PHP.B’s benefit is its increased ability to get through the vasculature into the brain,” says Deverman. “Once there, many AAVs, including AAV9 are quite good at delivering genes to neurons and glia.”
Gradinaru notes that since AAV-PHP.B is delivered through the bloodstream, it reaches other parts of the body. “Although in this study we were focused on the brain, we were also able to use whole-body tissue clearing to look at its biodistribution throughout the body,” she says.
Whole-body tissue clearing by PARS CLARITY, a technique developed previously in the Gradinaru lab to make normally opaque mammalian tissues transparent, allows organs to be examined without the laborious task of making thin slide-mounted sections. Thus, tissue clearing allows researchers to more quickly screen the viral vectors for those that best target the cells and organs of interest.
“In this case, the priority was to express the gene in the brain, but we can see by using whole-body clearing that you can actually have expression in many other organs and even in the peripheral nerves,” explains Gradinaru. “By making tissues transparent and looking through them, we can obtain more information about these viruses and identify targets that we might overlook otherwise.”
The biologists conducted follow-up studies up to a year after the initial injections and found that the protein continued to be expressed efficiently. Such long-term expression is important for gene therapy studies in humans.
In collaboration with colleagues from Stanford University, Deverman and Gradinaru also showed that AAV-PHP.B is better than AAV9 at delivering genes to human neurons and glia.
The researchers hope to begin testing AAV-PHP.B’s ability to deliver potentially therapeutic genes in disease models. They are also working to further evolve the virus to make even better performing variants and to produce variants that target certain cell types with more specificity.
Deverman says that the CREATE system could indeed be applied to develop AAVs capable of delivering genes specifically to many different cell types. “There are hundreds of different Cre transgenic lines available,” he says. “Researchers have put Cre recombinase under the control of gene regulatory elements so that it is only made in certain cell types. That means that regardless of whether your objective is to target liver cells or a particular type of neuron, you can almost always find a mouse that has Cre recombinase expressed in those cells.”
“The CREATE system gave us a good hit early on, but we are excited about the future potential of using this approach to generate viruses that have very good cell-type specificity in different organisms, especially the less genetically tractable ones,” says Gradinaru. “This is just the first step. We can take these tools and concepts in many exciting directions to further enhance this work, and we—with the Beckman Institute and collaborators—are ready to pursue those possibilities.”
The Beckman Institute at Caltech recently opened a resource center called CLOVER (CLARITY, Optogenetics, and Vector Engineering Research Center) to support such research efforts involving tissue clearing and imaging, optogenetic studies, and custom gene-delivery vehicle development. Deverman is the center’s director, and Gradinaru is the principal investigator.
Additional Caltech authors on the paper, “Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain,” are Sripriya Ravindra Kumar, Ken Y. Chan, Abhik Banerjee, Wei-Li Wu, and Bin Yang, as well as former Caltech students Piers L. Pravdo and Bryan P. Simpson. Nina Huber and Sergiu P. Pasca of Stanford University School of Medicine are also coauthors. The work was supported by funding from the Hereditary Disease Foundation and the Caltech-City of Hope Biomedical Initiative, a National Institutes of Health (NIH) Director’s New Innovator Award, the NIH’s National Institute of Aging and National Institute of Mental Health, the Beckman Institute, and the Gordon and Betty Moore Foundation.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!