If the changing seasons are making it chilly inside your house, you might just turn the heater on. That’s a reasonable response to a cold environment: switching to a toastier and more comfortable state until it warms up outside. And so it’s no surprise that biologists have long thought cells would respond to their environment in a similar way.
But now researchers at the California Institute of Technology (Caltech) are finding that cells can respond using a new kind of pulsating mechanism, instead of just shifting from one steady state to another and staying there. The principles behind this process are surprisingly simple, the researchers say, and could drive other cellular processes, revealing more about how the cells—and ultimately life—work.
In their experiment, the researchers studied how a bacterial species called B. subtilis responds to a stressful environment—for example, one without food. In such conditions, the single-celled organism activates a large set of genes that help it deal with hardship, by aiding cell repair for instance. Previously, biologists had thought the bacteria would handle stress by turning on the relevant genes and simply leaving them on until the stress goes away.
Instead, the researchers found that B. subtilis continuously flips these genes on and off. When faced with more stress, it increases the frequency of these pulses. The pulsating action is like switching your heater on full blast for a brief period every few minutes, and turning it on and off more frequently if you want the house to be warmer.
“It’s a very different view of how a cell can respond to a particular stress,” says James Locke, a postdoctoral scholar at Caltech. Locke and graduate student Jonathan Young are the lead authors on a paper describing this work, which was published in the October 21 issue of Science.
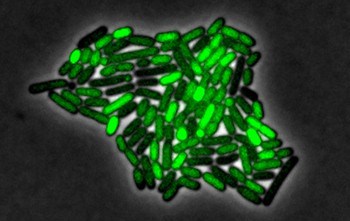
To make their finding, the researchers introduced a chemical to B. subtilis that inhibits the production of ATP, the energy-carrying molecules of cells. The team found that the stress induced by this chemical triggers interactions within a set of genes—collectively called a genetic circuit. This circuit, which contains a set of positive and negative feedback loops, generates sustained pulses of activity in a key regulatory protein called σB (“sigma B”). The researchers attached fluorescent proteins to the circuit, causing the cells to glow green when σB was activated. By making movies of the flashing cells, the team could then study the dynamics of the circuit.
The key to this pulsating mechanism is the variability inherent in how proteins are made, the researchers say. The number of copies of any specific protein in a given cell fluctuates over time. The bacterial gene circuit amplifies these molecular fluctuations, also called noise, to generate discrete pulses of σB activation. The stress also activates another key protein that modulates the pulse frequencies.
By turning a steady input (the stress) into an oscillating output (the activation of σB) the genetic circuit is analogous to an electrical inverter, a device that converts direct current (DC) into alternating current (AC), explains Michael Elowitz, professor of biology and bioengineering at Caltech, Howard Hughes Medical Institute investigator, and coauthor of the paper. “You might think you need some kind of elaborate circuitry to implement that, but the cell can do it with just a few proteins, and by taking advantage of noise.”
This work provides a blueprint for how relatively simple genetic circuits can generate complex and dynamic behaviors in individual cells, the researchers say. “We’re excited to think that similar mechanisms may occur in other cellular processes,” Locke says. “It’d be interesting in the future to see which aspects of this circuit architecture also appear in more complex systems, such as mammalian cells.”
“With this work and recent work in other systems, we’re starting to get a glimpse of just how dynamic cellular control systems really are,” Elowitz adds. “That’s something that was very difficult to see in the past.”
The other authors of the Science paper, “Stochastic pulse regulation in bacterial stress response,” are research technicians Michelle Fontes and Maria Jesus Hernandez Jimenez. The research was funded by the National Institutes of Health, the National Science Foundation, the Packard Foundation, the International Human Frontier Science Organization, and the European Molecular Biology Organization.
Written by Marcus Woo
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!