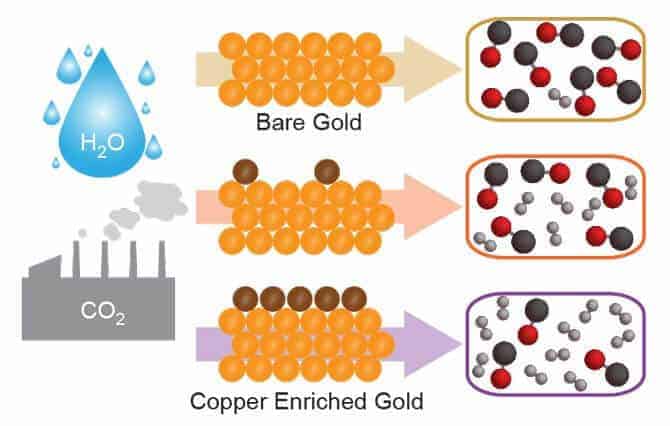
Scientists have developed a new recipe for creating synthesis gas mixtures, or syngas, that involves adding a pinch of copper atoms sprinkled atop a gold surface. The new material supports a room-temperature electrochemical reaction that can convert carbon dioxide and water into syngas, a mixture of carbon monoxide and hydrogen, and an important precursor in the production of chemicals and synthetic fuels.
The researchers say syngas can be converted downstream into small molecules, like ethanol, or larger hydrocarbons, such as those in gasoline, by fermentation or thermochemistry. Designing a material and a process that can easily control the composition of syngas would be an important improvement in reducing the environmental impacts of those industrial processes.
They describe their design in a paper recently published in the Journal of the American Chemical Society. The study was led by Peidong Yang, senior faculty scientist at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) Materials Sciences Division, and Edward Sargent, professor at the University of Toronto’s Department of Electrical and Computer Engineering.
“We know of no other single electrocatalyst that combines high production rates with such wide-ranging syngas composition control,” said Yang, who is also a professor of chemistry at the University of California, Berkeley. “Many processes that utilize syngas require different compositions of gas, so we wanted to create a family of electrocatalysts that can be easily tunable.”
The researchers found that they could control the amount of carbon monoxide and hydrogen generated by the electrocatalyst by adjusting the amount of copper atoms layered onto a nanostructured gold surface.
“The copper changes the strength with which CO2 binds with the surface,” said study lead author Michael Ross, a postdoctoral researcher in Yang’s lab. “A nanostructured surface that is primarily gold yields mostly carbon monoxide. To produce a mixture that is more hydrogen-rich, we add more copper.”
The researchers used X-ray photoelectron spectroscopy techniques at Berkeley Lab’s Molecular Foundry to quantify the amount of copper on the gold electrocatalyst needed to create different syngas mixtures. For example, a 1-atom-thick layer of copper covering the gold surface can produce a 2-to-1 mixture of hydrogen to carbon monoxide. When the gold is left unadulterated, the hydrogen-to-carbon monoxide mix is 1-to-10, demonstrating wide flexibility in syngas output.
“If these electrocatalysts could be scaled up to work in industrial reactors, we could make syngas using renewably generated electricity and CO2,” said Ross. “Syngas is currently being converted into methanol, diesel fuel, and other useful chemicals all over the world. This could make the production of these chemicals much more sustainable.”
This work in the Catalysis Research Program at Berkeley Lab’s Chemical Sciences Division was supported by DOE’s Office of Science and the Canadian Institute for Advanced Research. The Molecular Foundry is a DOE Office of Science User Facility.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!