Sepsis, the body’s response to severe infections, kills more people than breast cancer, prostate cancer and HIV/AIDS combined. On average, 30 percent of those diagnosed with sepsis die.
A study conducted by Jamey Marth, director of UC Santa Barbara’s Center for Nanomedicine and professor of the Sanford-Burnham Medical Research Institute, reports a new method to increase survival in sepsis. The results appear today in the Proceedings of the National Academy of Science.
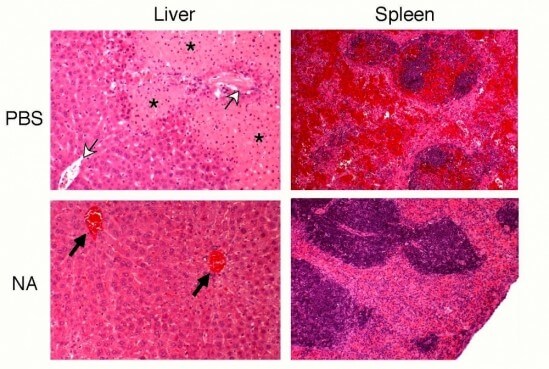
Building on earlier work in which Marth’s team revealed the biological purpose of the Ashwell-Morell receptor (AMR) in the liver, the new discovery not only describes the AMR’s protective mechanism, but also outlines a way to leverage it for therapeutic use. Sepsis often triggers widespread blood coagulation and thrombosis, which can lead to organ failure and death.
The researchers found that the AMR protects the host by the rapid removal of the prothrombotic components normally present in the bloodstream, including platelets and specific coagulation factors that contribute to the formation of blood clots. The study elucidates this mechanism of AMR function in mitigating the lethal effects of excessive blood coagulation and thrombosis in sepsis.
The key is neuraminidase, an enzyme that is present in many pathogenic microorganisms, such as Streptococcus pneumoniae, the bacteria used in this study, which remains one of the top five causes of death worldwide. Pathogens use neuraminidase to get into cells, but once the pathogen enters the bloodstream, the enzyme then remodels the surface of platelets and other glycoproteins in circulation. This remodeling signals the AMR to remove those platelets and coagulation factors before they have a chance to contribute to the lethal coagulopathy of sepsis.
“It’s a highly conserved protective mechanism never before identified,” said Marth, who is also Carbon Professor of Biochemistry and Molecular Biology and Mellichamp Professor of Systems Biology at UCSB. “The host has evolved this protective mechanism over millions of years as a way to compensate for the lethal impact of the pathogen on our coagulation system.”
The scientists wondered what would happen if they could pre-activate and augment AMR function in the early phases of sepsis. To answer that question, they infected mice with Streptococcus pneumoniae and then gave them a single dose of neuraminidase. “We were able to increase survival twofold,” said Marth. “It’s remarkable, and because we see the same mechanism active in human sepsis there is excitement by the potential of this approach to save millions of lives.”
In teasing out the details of the AMR’s protective mechanism, Marth and his colleagues learned that the receptor has the capability to selectively identify and remove certain blood components that could harm the host if they contributed to blood clotting in sepsis.
Although some scientists have suggested that little may be gained from research on sepsis in non-human species, the study by the Marth team discloses a mechanism of host protection that is conserved through mammalian evolution and which can be easily manipulated. The fact that this mechanism is imperceptible to studies of genomic variation and gene expression may explain why others have not discovered it earlier. “Much of biomedical research is focused on the gene. In our research, it was the study of metabolism that provided the key,” explained Marth.
“Because it appears that the same protective mechanism exists in humans, investors have already contacted us about moving this forward into clinical trials,” Marth said. “It’s estimated that 50 to 100 million people around the world have sepsis each year, and we can now imagine a simple effective treatment consisting of a non-refrigerated enzyme mixed with saline, placed in a syringe and injected intravenously. This has the potential to translate into saved lives among those in the developed and undeveloped world.”
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!