Engineering researchers at Rensselaer Polytechnic Institute have developed a new type of rechargeable lithium battery with components made entirely of carbon. Unlike the lithium-ion batteries currently sold around the world to power smart phones, laptops, and countless other devices, this new battery is made without the toxic metal cobalt.
The new technology, detailed in a research paper published this week in the journal Nature Communications, pairs an anode and cathode made from the nanomaterial graphene with metallic lithium to create an energy storage device. This research could lead to a new generation of batteries with significantly higher energy density, and that are nontoxic, easy to manufacture, and inexpensive to recycle.
“In this study, we show how to make a new type of battery using graphene, as well as an unlikely component – metallic lithium,” said Nikhil Koratkar, the John A. Clark and Edward T. Crossan Professor of Engineering at Rensselaer, who led the study. “Metallic lithium is avoided in lithium-ion batteries due to its tendency to form dendrites which are considered unsafe, but we show that metallic lithium trapped within a porous graphene structure is safe and does not form dendrites. The result is an all-carbon lithium battery that offers up to three times higher energy density than conventional batteries. It is also more environmentally friendly, and with its simplified chemistry could be easier and less expensive to mass produce.”
See the study, titled “Defect-induced plating of lithium metal within porous graphene networks,” online at: http://go.nature.com/htWO9h
Lithium battery technology dates back to the 1970s, and some of the very first iterations used a metal lithium cathode. Energy is stored by moving lithium ions back and forth between the battery’s cathode and an anode made of graphite. This results in a flow of electrons, which is harvested and put to use as electricity.
As the lithium gets cycled back and forth between the anode and cathode, it would settle and form dendrites — small, sharp towers on the cathode surface — that over time grow in size and eventually pierce the membrane separating the cathode and anode, causing the battery to fail. Even though lithium metal has an attractive energy density, this shortcoming led to serious safety concerns. As a consequence, the battery industry has avoided the use of metallic lithium and has resorted to storing elemental lithium in a matrix material such as cobalt-oxide or iron-phosphate. Atoms of cobalt or iron in the matrix bond with lithium and prevent lithium metal from forming, thereby solving the dendrite issue.
In most lithium-ion batteries sold today, the cathode is made from lithium cobalt oxide and the anode is made from graphite, comprised of many ultra-thin layers of graphene. When charging, lithium ions seek out the edges of the anode, and work their way inside between the layers of graphene. This process, called intercalation, creates no bonds between the elemental lithium or between the lithium and graphene, which limits the energy density of the overall battery.
In place of graphite, Koratkar and his research team developed an anode made from thermally shocked graphene. This process is a way to intentionally create cracks, holes, voids, and other defects in the graphene. In previous studies, the researchers have shown that these defects enable lithium ions to more rapidly make their way into the anode, leading to shorter charge times.
In this study, however, they looked at the interaction of elemental lithium with such defect sites in the graphene. They found that lithium ions are strongly attracted to defect sites, which creates a very high local concentration of lithium near the defects. This initiates the formation, or “plating,” of metallic lithium at the defect sites, which significantly increases the energy density of the battery. Such graphene-lithium metal composites can be used as the cathode, eliminating the need for toxic metals or complicated chemistries associated with lithium cobalt oxide or lithium iron phosphate.
However, what surprised the research team was that even after thousands of charge-recharge cycles, as the metallic lithium transitioned back and forth between the anode and cathode, no significant dendrites formed.
“We discovered that the porous graphene network acts as a caged entrapment for lithium metal that prevents dendritic growth, facilitating extended cycling of the electrode,” Koratkar said. “The result is a device with excellent energy density and stable performance. We are excited about its potential as a new type of battery.”
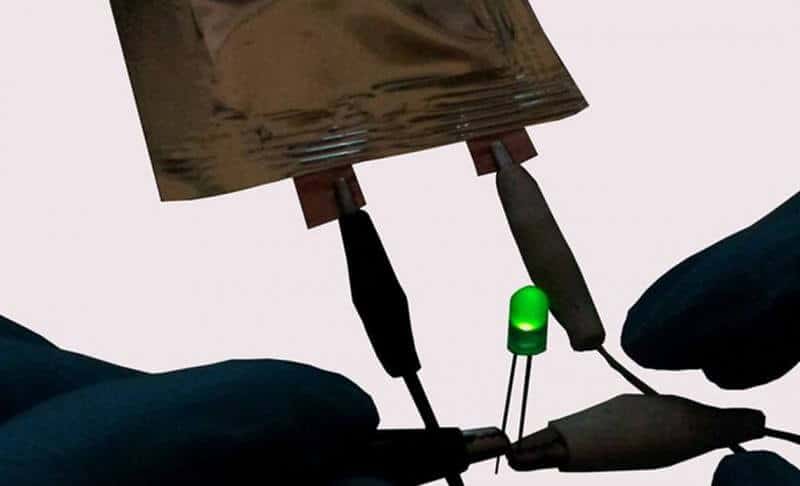
When compared to traditional graphitic anodes and conventional lithium cobalt oxide cathodes, the new graphene-lithium metal composite electrode provides higher charge storage capacity and up to three times higher energy density. Extended testing for over 1,000 charge-discharge cycles showed highly stable performance. Importantly, even after 1,000 cycles there was no indication of any significant dendritic structures, since the lithium metal is caged within the pores of the porous graphene network structure. To demonstrate the concept, the researchers built a cell using graphene electrodes and used it to power a commercial LED device.
Along with Koratkar, co-authors of the paper are: graduate students Rahul Mukherjee, Abhay Thomas, and Eklavya Singh, and visiting scientist Osman Eksik, of the Rensselaer Department of Mechanical, Aerospace, and Nuclear Engineering; graduate student Dibakar Datta of Brown University; and postdoctoral scientist Junwen Li and Professor Vivek Shenoy of the University of Pennsylvania.
Koratkar’s graduate student and first author of the paper, Mukherjee, was a finalist in the 2014 MIT-Lemelson National Collegiate Student Prize Competition in the graduate student category and presented his research at the Massachusetts Institute of Technology earlier this month. With fellow student Eklavya Singh, Mukherjee won the “best of the best” grand prize at the spring 2014 Change the World Challenge competition at Rensselaer.
This research was supported by the National Science Foundation, as well as the John A. Clark and Edward T. Crossan Professorship at Rensselaer, and a Bob Buhrmaster ’69 grant from the Severino Center for Technological Entrepreneurship and Lally School of Management at Rensselaer.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!

unsafe disposal of batteries had led to many infections around the world and researchers have been prompted to find ways to reduce the effects. it is a good thing that they have managed to remove the toxic cobalt in batteries such that they are safer to dispose off. However, in the same process they have also managed to make the batteries easily recyclable and they produce more energy for longer periods of time.
In the world we are living ,the one thing that drives the world around is energy.Whether it is sun-energy ,electricity or even the energy in food that our bodies need in order to function.Batteries is basically small stored electricity cells which are important in powering our cars,laptops,and so forth.Without electricity we cannot function or produce work,for example a business entrepreneur has to present his new business ideas to the company using his laptop and projector.A car cannot start without a battery ,it is as simple as that.This new carbon battery provides us with “clean” or “green” energy because it takes carbon which is a pollutant and it is converted to give us energy.It is very difficult recycling batteries due to their chemical components and that some countries do not have the needed technology in recycling batteries .This technology is sustainable and is definitely a step forward in protecting our precious earth from pollinating.For further information on battery recycling factories in America ,http://www.americasbatteryrecyclers.com/technology.html
I have personal experience with traditional rechargeable lithium batteries as I have known them not have a particularly long lifespan and can fail at the most inconvenient times. The promise that the rechargeable carbon batteries present is very appealing to me as they would provide a greater energy output and are less prone to failing after a certain number of recharging them. They are also due to them being a cheaper alternative and provide a greater value for money that their lithium counterparts.
this new technology appeals to me for the sole fact that i can produce the raw ingredients to make use of these batteries as carbon is such an abundant organic material on earth.
the prospect of carbon,one of the world most problematic byproducts of our industrial activities can be utilized as an energy source is very appealing to me cause of the massive destruction this century has witnessed due to air pollution.
Lithium batteries have an inherent unstable nature that manufacturers were well aware of when the first batteries were rolled out in the 70’s. This is due to the battery accumulating metallic particles within the cell and therefore forming small structure’s that can pierce the shell called dendrites. They have been known to cause an increase in the temperature of the cell. This process is called thermal runaway which can cause the battery to explode. The fact that the carbon batteries don’t share this same attribute is comforting as a greater level of safety is archived when using electrical devices such as cellophanes and laptops.
Modern society has remarkably high energy needs of which are pushing the earth and its resources to its limits. Breakthroughs such as these go without saying that are very welcome. Carbon being the main component of all organic compounds and a bye product of most of our industrial activities make it easily available to be utilized in the production of these revolutionary batteries. Nations such as the Peoples Republic Of China would greatly benefit from this technology as they have a huge crisis on their hands concerning the large accumulation of carbon dioxide and other air pollutants in their skies have made it the most polluted skies in the world. A study done by then W.H.O (World Health Organization) in 2010 revealed that 1.2 million premature deaths could be linked directly to the pollution of their skies. Application of this technology can potentially solve two of our society’s most daunting problems.
It is great to hear about the new battery which not only contains components made entirely of carbon, but also that it is rechargeable. I recently read an article which mentioned that South Africans throw away about 50 million batteries annually. It goes on to say that a single AAA battery can pollute 500 litres of water for 50 years (Dianne Bayley, http://www.africanenvironment.co.za). I live in South Africa and find this shocking because most of our batteries are not even recycled. They are simply dumped in massive landfill sites where the toxic metal cobalt can seep into the ground water and contaminate it. The introduction of this revolutionary new battery into the South African market could make a huge contribution in the preservation of our groundwater resources.