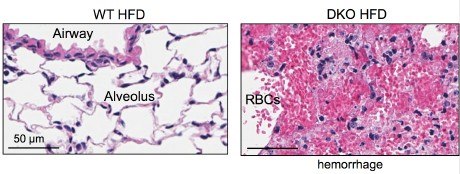
When Cornell researchers fed high-fat Western diets to mice engineered without key immune system receptors to recognize pathogens, the mice died from lethal lung damage.
But upon closer inspection, the cause of death in these mice may have resulted from a proliferation of a group of common gut bacteria, which released toxins that accumulated in the blood stream to deadly levels and led to lung hemorrhaging.
The findings, published June 18 in the journal Cell Reports, offer clues to a little-known area of research: how Western diets, which have driven an epidemic of obesity and metabolic syndrome, increase mortality in humans.
More work is needed to better understand the relationships between high-fat diets, immunity and gut bacteria, but the researchers believe they have opened an important new avenue for research.
“With this study, the role of gut bacteria in the mortality of obese patients can be explored,” said Ling Qi, associate professor of nutritional sciences and the paper’s senior author. Yewei Ji, a postdoctoral fellow, and Shengyi Iris Sun, a graduate student, both in Qi’s lab, are co-lead authors of the paper.
The researchers were initially interested in studying how obesity can lead to insulin resistance (or metabolic syndrome) and accompanying inflammation, which place a person at risk for diabetes.
Qi and colleagues attempted to reduce inflammation, which is an immune response, by engineering mice without so-called “toll-like receptors” that recognize pathogens. Both control mice and engineered mice were fed high-fat diets, and while all the mice got fat, the engineered mice died of pulmonary damage. The researchers found that antibiotics prevented death.
“We know that [toxins from] gut bacteria are sufficient to cause pulmonary damage,” Qi said. “We don’t know how the bacteria becomes prolific” in the engineered mice, he added.
Qi and colleagues also put the control mice in the same cage with the engineered mice. In doing so, the gut bacteria from the engineered mice colonized in the control mice (as mice are known to eat feces), leading to death in control mice as well. The researchers also filtered the fecal content in the engineered mice, removing all the solid materials and bacteria and leaving a fluid that contained toxins from the gut bacteria. When this fluid was injected into the control mice, the mice developed lung injuries as well, indicating that these toxins are likely playing a role in the lung damage.
Interestingly, engineered mice that were fed low-fat diets lived. This finding suggests that the mortality is diet-dependent.
“We believe that bacteria proliferation is related to high-fat diets and a lack of immune system surveillance in the engineered mice,” said Ji. “The results of this study may help dietary choices in immune-compromised patients such as those under radio- and chemotherapy,” added Sun.
In future research, Qi and colleagues will try to learn more about these gut bacteria, identify the bacterial toxins and identify antibiotics that are effective against these bacteria.
Gerald Duhamel, professor of biomedical sciences, and Ruth Ley, associate professor of microbiology and genetics, were co-authors on the paper. Postdoctoral fellow Angela Poole (in Ley’s lab) and graduate students Hana Kim (in Qi’s lab) and Julia Goodrich (in Ley’s lab) contributed to the study.
The study was funded by the David and Lucile Packard Foundation, the National Science Foundation, the Juvenile Diabetes Research Foundation, the American Diabetes Association and the National Institutes of Health.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!