Inhibiting a nuclear receptor in the gut could lead to a treatment for a liver disorder that affects almost 30 percent of the Western world’s adult population, according to an international team of researchers.
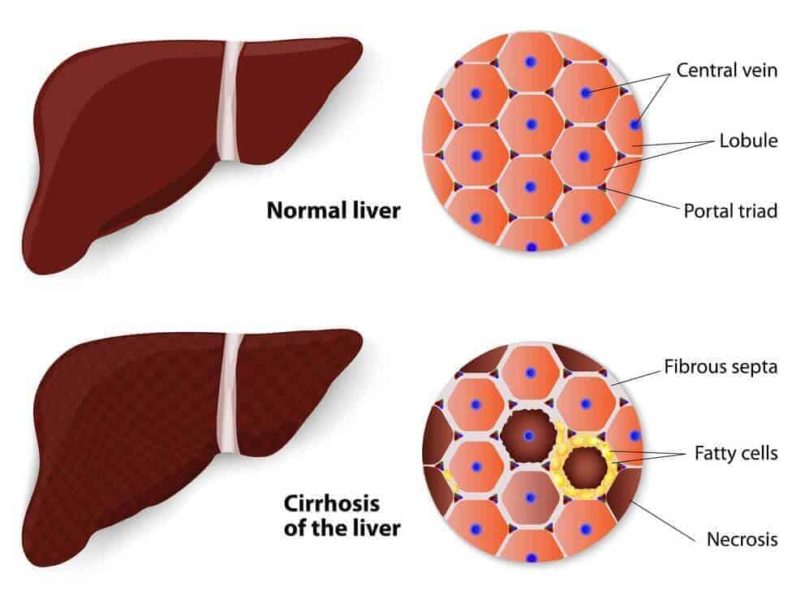
The researchers found that tempol, an antioxidant drug, and antibiotics can treat and prevent nonalcoholic fatty liver disease in mice that were fed a high-fat diet. Nonalcoholic fatty liver disease — NAFLD — is a build up of fat in liver cells that disrupts liver function and, if left untreated, can lead to liver failure.
“The effects of this disease are staggering in the United States and around the world,” said Andrew Patterson, assistant professor of molecular toxicology, Penn State. “Some patients with NAFLD can develop a range of health problems, such as steatohepatitis, fibrosis and cirrhosis that, if it gets this far, may require a liver transplant.”
The key mechanism for the positive effect seen from the drugs tested is that these drugs indirectly inhibited the intestinal farnesoid X receptor — FXR — in the mice, according to Patterson. FXR is a transcription factor that controls bile acid synthesis and transport in the liver and intestine.
“What our team found is that, if we genetically disrupt FXR expression in the intestinal epithelium, or inhibit it with tempol or antibiotics, we can prevent the development of NAFLD and obesity,” Patterson said. “Now, this is in mice, so the next thing is to continue to take the next steps and, eventually, to see if FXR might be a suitable target in humans.”
Patterson said that, according to this study and earlier research, tempol and antibiotics appear to reduce some types of Lactobacillus — an intestinal bacteria that converts lactose and other sugars into lactic acid. When Lactobacillus levels decrease, a bile acid — tauro-beta-muricholic acid — increases. The increase of this bile acid then directly inhibits FXR — farnesoid X receptor, which regulates the metabolism of bile acids, fats and glucose in the body, according to the researchers.
This study is another indication of the complex role that gut microbes play in human health, as well as how they may serve as possible targets for treating metabolic disorders, according to the researchers, who report their findings in the current issue of The Journal of Clinical Investigation.
These microbes make up the microbiome, the community of biological microorganisms that live in and on the human body. Changing the microbiome can help promote health and may also be associated with disease, Patterson said.
“The microbiome is incredibly complex and has been the subject of intense study in the last few years, due to the development of deep sequencing and most importantly, due to the ability to analyze this complex data,” said Patterson. “What is exciting about this study, in particular, is that we feel like we are taking another small step in shedding some light on how diet and drugs can modulate bacteria and how these organisms modify their environment.”
However, Patterson added, more work will be required to determine whether these findings are applicable to treat humans. While the digestive systems of mice and humans are similar, there are important differences.
“Lactobacillus, themselves, appear to differ in both organization and numbers in a mouse’s digestive system compared to a human’s, for example,” Patterson said.
The researchers will also study possible side effects from the modulation of gut bacteria and FXR by drug treatment.
“We are proceeding cautiously,” said Patterson. “It’s very easy to over-hype this type of research, as we feel the research is just in its infancy.”
Patterson worked with Frank J. Gonzalez, laboratory metabolism chief; Changtao Jiang and Cen Xie, postdoctoral scholars; Kristopher W. Krausz, research biologist; Fei Li, Yunpeng Qi, Shogo Takahashi, Zhong-Ze Fang, Naoki Tanaka, all visiting Fellows, all of the National Cancer Institute; Limin Zhang, visiting scientist; Robert G. Nichols, graduate student; Jingwei Cai, graduate student; Dhimant Desai, associate professor of pharmacology; Shantu G. Amin, professor of pharmacology, all of Penn State Hershey College of Medicine; and Istvan Albert, associate professor of biochemistry and microbiology, Penn State.
The National Institutes of Health, the National Institutes of Environmental Health Sciences and the Pennsylvania Department of Health, using Tobacco CURE funds, supported this work.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!

If someone’s liver doesn’t work, we blame it on the genes; if someone’s brain doesn’t work properly, we blame the school. It’s actually more humane to think of the condition as genetic. For instance, you don’t want to say that someone is born unpleasant, but sometimes that might be true.