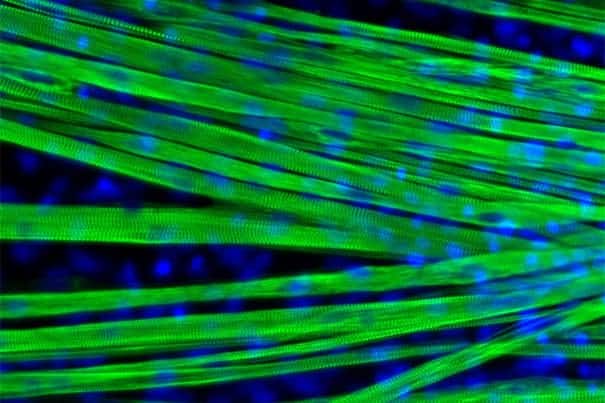
Skeletal muscle is one of the most abundant tissue types in the human body, but it has proven difficult to produce in large quantities in the lab. Unlike other cell types, such as heart cells, neurons, and cells found in the gut, previous attempts to efficiently and accurately derive muscle cells from precursor cells or culture have not been fruitful.
In a new study published this week in Nature Biotechnology, investigators from Harvard-affiliated Brigham and Women’s Hospital (BWH) report that by identifying and mimicking important developmental cues, they have been able to drive cells to grow into muscle fibers, producing millimeter-long fibers capable of contracting in a dish and multiplying in large numbers. This new method of producing muscle cells could offer a better model for studying muscle diseases, such as muscular dystrophy, and for testing potential treatment options.
Previous studies have used genetic modification to create small numbers of muscle cells in the lab, but the researchers, led by investigators from BWH and the Harvard Stem Cell Institute, wanted a technique that would allow them to grow large numbers of muscle cells efficiently for use in clinical applications.
“We took the hard route: We wanted to recapitulate all of the early stages of muscle cell development that happen in the body and re-create that in a dish in the lab,” said corresponding author Olivier Pourquie of Harvard Medical School’s Department of Genetics and BWH’s Department of Pathology. “We analyzed each stage of early development, and generated cell lines that glowed green when they reached each stage. Going step by step, we managed to mimic each stage of development and coax cells toward muscle cell fate.”
The team found that a combination of secreted factors that are important at early embryonic stages are also essential for stimulating differentiation — the specialization of stem cells into particular cell types — in the lab. Using just the right recipe for differentiation, the team was able to produce long, mature fibers in a dish, derived from mouse or human pluripotent stem cells. They also cultured stem cells from the mouse model of Duchenne muscular dystrophy, observing the striking branched pattern that dystrophin-deficient muscle fibers show in the body.
The research team also was able to produce more immature cells known as satellite cells that, when grafted into a mouse model of Duchenne muscular dystrophy, produced muscle fibers. Further studies will be needed to determine if the new strategy could be optimized to develop cell therapies for treating degenerative diseases in humans.
In addition to developing a better model for Duchenne muscular dystrophy, the new protocol also may be useful for studying other muscle diseases, such as sarcopenia (degenerative muscle loss), cachexia (wasting away of muscle associated with severe illness), and other muscular dystrophies.
“This has been the missing piece: The ability to produce muscle cells in the lab could give us the ability to test out new treatments and tackle a spectrum of muscle diseases,” said Pourquie.
This work was supported by the European Research Council, the Stowers Institute for Medical Research, the Howard Hughes Medical Institute, PluriMes, and by a strategic grant from the French Muscular Dystrophy Association.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!