Researchers have developed a procedure to mimic in laboratory experiments a form of brain trauma commonly seen in combat veterans, and findings suggest a new diagnostic tool for early detection and a potential treatment.
About one in five wounded soldiers suffers from traumatic brain injury and an estimated 52 percent of those injuries are blast-induced neurotrauma. A subclass called mild blast-induced neurotrauma is particularly difficult to diagnose because people who have it often display no obvious motor impairment or other neurological symptoms, said Riyi Shi, a professor in Purdue University’s Department of Basic Medical Sciences, College of Veterinary Medicine, and Weldon School of Biomedical Engineering.
“Many times they don’t even realize they’ve been injured, and this is particularly alarming because these injuries have been linked to severe long-term psychiatric and degenerative neurological dysfunction,” he said. “The underlying mechanisms of injury remain poorly understood, impeding development of diagnostic and treatment strategies.”
The initial injury is caused by the shock wave from explosions. However, researchers believe secondary damage takes place in the days and weeks that follow the initial injury, and this secondary damage might be treatable.
The researchers have developed a method to mimic mild blast-induced neurotrauma in laboratory rats, representing a new strategy to establish a clinically relevant “animal model” that recreates typical human symptom profiles. This model can be used to study the effects and pinpoint mechanisms responsible for ongoing damage that occurs following the initial injury, Shi said.
Findings, which appeared on Aug. 21 in the Journal of Neurosurgery, suggest a simple urine test could be used to diagnose the injury, and damaging effects might be alleviated through drug therapy that reduces the concentration of a toxic compound produced by traumatized cells.
“Early detection and intervention could potentially mitigate or prevent delayed onset development of significant neurological dysfunction,” Shi said.
The paper was authored by graduate students Michael Walls, Nicholas Race, Lingxing Zheng, Sasha M. Vega Alvarez, Glen Acosta, Jonghyuck Park and Shi.
The research shows evidence of brain inflammation that may indicate ongoing damage, potentially leading to altered brain function and degenerative diseases.
“We detected structural and biochemical brain damage without obvious motor or cognitive deficits,” Shi said. “These findings highlight the difficulty and importance of early detection, indicating missed early diagnosis and subsequent lack of intervention could lead to serious long-term consequences.”
A neurotoxin called acrolein is produced within the body after nerve cells are damaged and has been shown to lead to continued damage. However, the concentration of acrolein could be reduced using the drug hydralazine, which has been approved by the U.S. Food and Drug Administration for hypertension, he said.
The drug has been shown to be effective in reducing acrolein levels in previous research led by Shi, who is working to develop a low-dose version for that purpose in humans.
New findings indicate elevated levels of acrolein in brain tissue and in urine from research animals lacking neurological signs of damage. Acrolein concentrations were three times the normal level the first day of the experiment and remained elevated five days later. The findings suggest urine tests showing elevated acrolein might indicate trauma despite the lack of symptoms following mild blast injury. Treatment at this point could reduce the risk of developing chronic neurological diseases, said Shi, who is a member of the International Brain Mechanics and Trauma Lab, a newly established initiative aiming to gather multidisciplinary expertise for the study of brain mechanics in trauma and diseases.
The research was supported in part by the Indiana State Department of Health (Grant #204200 to RS), National Institutes of Health (Grant # NS073636 to RS), and Indiana CTSI Collaboration in Biomedical Translational Research Pilot Program Grant (Grant #RR025761 to RS). Funding for the LSM710 was provided by NIH NCRR Shared instrumentation Grant 1 S10 RR023734-01A1.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!

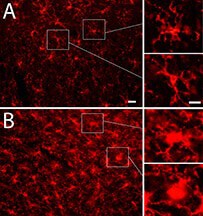
I suppose this gives a bit of credence to my hypothesis the damage in blast-induced brain trauma is caused by iron released from extravasated red blood cells, since the drug hydralazine is an iron chelator.
“We studied the effects of another PHi which is also an iron chelator, hydralazine”