Could the future of chemical manufacturing be smaller, faster, and greener? A significant advancement from researchers at UC Santa Barbara, UCSF, and the University of Pittsburgh suggests exactly that.
In a study published in Science, the research team has developed a revolutionary workflow for designing enzymes from scratch rather than modifying existing ones. These custom catalysts could transform pharmaceutical development, materials science, and industrial chemistry by enabling more efficient, selective, and environmentally friendly processes.
“If people could design very efficient enzymes from scratch, you could solve many important problems,” said UCSB chemistry professor Yang Yang, a senior author on the paper.
Building Better Catalysts Through Design
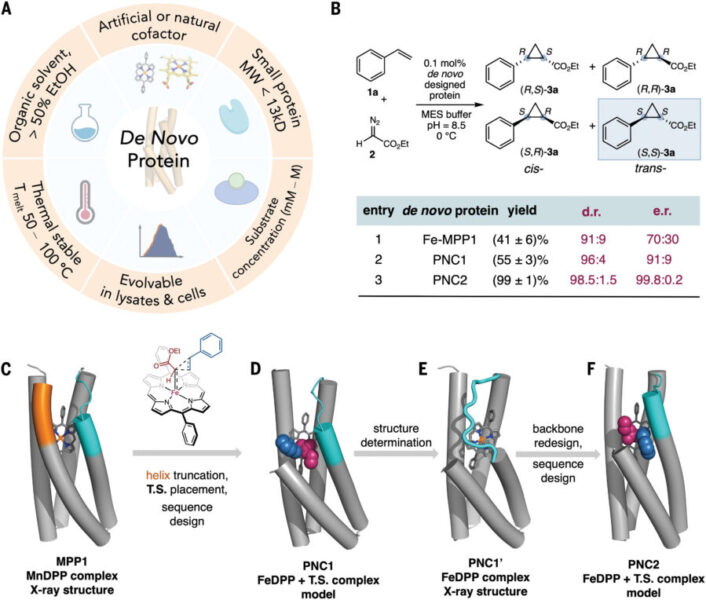
The innovative approach centers on creating “de novo” proteins—completely new enzymes built from amino acid building blocks rather than modified versions of existing proteins. The team focused specifically on catalyzing reactions for which natural enzymes perform poorly or don’t exist at all.
These custom-designed enzymes, nicknamed “NovoChromes,” demonstrated remarkable abilities:
* Creating carbon-carbon and carbon-silicon bonds with precision
* Operating at temperatures up to 100°C
* Maintaining stability in environments containing up to 70% organic solvents
* Working faster than highly evolved natural enzymes
* Maintaining high selectivity despite their smaller size
“For fundamental research, chemists and biologists have long been hoping to have the ability to design enzymes from scratch,” Yang noted.
The Design Process: AI Meets Chemical Intuition
How does one build an enzyme from nothing? The team started with a simple helical bundle protein as their framework. They then employed cutting-edge artificial intelligence methods to design specific sequences of amino acids that would transform this basic structure into a powerful catalyst.
The process wasn’t straightforward, though. Initial designs showed promise but lacked optimal performance.
“The earlier variants were reasonable catalysts, but they were not the best because the efficiency and selectivity were modest,” Yang explained.
After analyzing their initial designs using X-ray crystallography, the researchers identified a structural flaw—a “disorganized loop” that needed refinement. This illustrates how even with advanced AI tools, human expertise remains essential.
“In other words, although AI-based protein design methods are very useful, to have very good catalysts we still have to use our in-house algorithm and our chemical intuition to get everything done the right way,” Yang said.
Record-Breaking Performance
The refined design process led to enzymes with truly impressive capabilities. One NovoChromes variant achieved a turnover frequency of 290,000 reactions per hour—comparable to natural enzymes that have been fine-tuned by billions of years of evolution.
When tested across a range of substrates with varying structures, the engineered enzymes maintained excellent performance. One particularly notable achievement was the creation of a cyclopropane with adjacent quaternary-tertiary stereocenters—a challenging molecular arrangement important in pharmaceutical development.
Evolution Meets Design
The team also demonstrated that their artificial enzymes could be further improved through directed evolution—a process of introducing mutations and selecting the most effective variants.
Through this process, they created an enzyme that could form carbon-silicon bonds with unprecedented efficiency. The evolved enzyme achieved a turnover number of 32,990—far surpassing previous catalysts for the same reaction.
Molecular simulations revealed the secret to this improved performance: the evolved enzymes had more rigid, preorganized active sites that held the reacting molecules in optimal positions.
Interestingly, one key mutation involved introducing a proline—an amino acid that typically disrupts helical structures—into a helix. This counterintuitive change helped the enzyme better match the contour of its substrates.
A Greener Chemistry Future
Beyond their impressive catalytic abilities, these enzymes offer significant environmental benefits. Traditional chemical manufacturing often relies on toxic solvents, hazardous metals, and harsh conditions.
“If you really understand the design principles, then you can build a protein catalyst to use whatever cofactors you would like to use, and to achieve challenging transformations in water, the greenest solvent, as the reaction medium,” Yang said.
This approach could lead to more sustainable chemical processes, potentially reducing pollution and energy consumption while improving efficiency and product purity.
The research team is now exploring ways to create even simpler, smaller enzymes with similarly high activity, and to generate enzymes that operate via mechanisms not previously known in nature.
As this technology develops, we may see a transformation in how pharmaceuticals, materials, and other chemicals are manufactured—a change that could benefit both industry and the environment.
Research in this study was conducted by Kaipeng Hou, Wei Huang, Miao Qui, Thomas H. Tugwell, Turki Alturaifi, Yuda Chen, Xingjie Zhang, Lei Lu, and Samuel I. Mann.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!