South Korean researchers have discovered a promising new approach to potentially prevent or treat Alzheimer’s disease by targeting the connection between herpes virus infection and brain inflammation.
The study, published in Theranostics, demonstrates how a new drug candidate called ALT001 effectively stops herpes simplex virus type 1 (HSV1) from impairing brain immune cells and enhances their ability to clear harmful protein accumulations associated with neurodegenerative diseases. This dual-action mechanism—simultaneously fighting viral infection while restoring normal brain immune function—represents a significant advance in understanding how infections might contribute to Alzheimer’s disease and offers a potential new treatment pathway for affected patients.
The research, led by Dr. Ok Sarah Shin from Korea University College of Medicine and Professor Jean-Ho Yun from Dong-A University College of Medicine, provides compelling evidence that viral infections may play a more significant role in neurodegenerative disorders than previously recognized. Their work specifically focuses on microglia, the brain’s immune cells, which play a crucial role in removing toxic proteins like amyloid beta (Aβ) that accumulate in Alzheimer’s disease.
How Herpes Virus Sabotages Brain Immune Cells
The team’s investigation revealed a complex chain of events by which HSV1 infection compromises brain health. Using multiple experimental models—including mouse cells, human stem cell-derived microglia, and sophisticated brain organoid models—the researchers tracked exactly how the virus disrupts normal immune function.
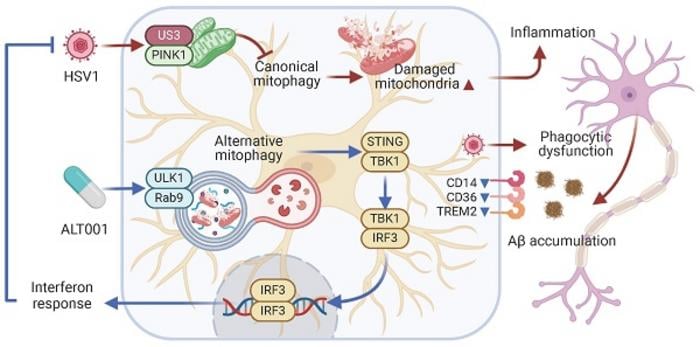
Their findings showed that HSV1 specifically targets a critical cellular cleaning process called mitophagy, which normally removes damaged mitochondria (the cell’s energy-producing components). The virus uses a protein called US3 to interfere with this cleaning process, leading to an accumulation of dysfunctional mitochondria that trigger inflammation and impair normal cell function.
This disruption creates a cascade of harmful effects. Infected microglia shift into an inflammatory state, producing higher levels of inflammation-promoting molecules like IL-6, IL-1β, and TNF-α. Simultaneously, the virus dramatically reduces the ability of these immune cells to perform their normal cleanup duties—particularly their capacity to clear away Aβ protein aggregates associated with Alzheimer’s disease.
ALT001: Restoring Cellular Cleanup Mechanisms
The research team discovered that the experimental drug ALT001 activates an alternative mitophagy pathway that bypasses the viral blockade. This drug works through a mechanism called ULK1/Rab9-dependent alternative mitophagy, which essentially provides a “backup” cleaning system when the primary mitophagy pathway is compromised.
When infected cells were treated with ALT001, the researchers observed several remarkable effects:
- Restored mitochondrial structure and function in infected cells
- Significant reduction in HSV1 replication and viral spread
- Enhanced interferon responses, boosting antiviral immunity
- Reduced inflammatory cytokine production
- Restored ability of microglia to engulf and remove Aβ protein aggregates
- Prevented neuronal damage in cell co-culture and brain organoid models
Transcriptomic analysis further revealed that ALT001 treatment shifted infected microglia from a disease-associated inflammatory state back toward a normal homeostatic condition, essentially “resetting” these immune cells despite ongoing viral pressure.
A New Paradigm for Understanding Alzheimer’s Disease
This research adds significant weight to the emerging theory that infections may contribute to or accelerate neurodegenerative diseases like Alzheimer’s. Previous epidemiological studies had shown associations between HSV1 infection and Alzheimer’s risk—with over 70% of late-onset Alzheimer’s cases showing evidence of latent HSV1 infection—but the precise mechanisms remained unclear.
“This study is highly significant as it not only demonstrates at a molecular level that viral infection can worsen neurodegenerative diseases, including Alzheimer’s, but also presents a new treatment strategy,” stated Professor Shin. “In particular, identifying the impact of HSV-1 infection on mitophagy in microglia is a distinct achievement compared to previous neuron-focused research. ALT001 could be applied in the future to treat various viral neurological disorders.”
Beyond Alzheimer’s: Broader Applications
The implications of this research extend beyond Alzheimer’s disease. The team’s findings suggest that ALT001 might offer potential therapeutic benefits for other conditions where impaired mitophagy or viral infections play a role in disease pathology.
Interestingly, ALT001 showed more potent antiviral effects against HSV1 than acyclovir, a commonly used antiviral medication, in laboratory testing. This raises the possibility that ALT001 could potentially serve as a treatment for herpes simplex encephalitis, a serious brain infection caused by HSV1.
While clinical trials will be necessary to confirm the safety and efficacy of ALT001 in humans, this research opens promising new avenues for treating neurodegenerative conditions by addressing both viral triggers and immune dysfunction simultaneously. As evidence continues to mount connecting infections to neurodegenerative diseases, this approach might ultimately change how we conceptualize and treat conditions like Alzheimer’s disease in the future.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!