Northwestern University scientists have developed an innovative nanotherapy that directly targets the root cause of Alzheimer’s disease and other neurodegenerative conditions.
The treatment, which essentially acts as a “clean-up crew” for misfolded proteins in the brain, dramatically improved neuron survival in laboratory tests. This approach, published Wednesday in the Journal of the American Chemical Society, could potentially slow disease progression by trapping toxic proteins before they can damage brain cells.
Targeting the Destructive Proteins at Their Source
In diseases like Alzheimer’s and ALS, proteins misfold and clump together around neurons, ultimately leading to cell death. Rather than attempting to treat symptoms after damage occurs, this new approach intervenes at an earlier stage in the disease process.
“Our study highlights the exciting potential of molecularly engineered nanomaterials to address the root causes of neurodegenerative diseases,” explained Northwestern’s Samuel I. Stupp, the study’s senior author. “In many of these diseases, proteins lose their functional folded structure and aggregate to make destructive fibers that enter neurons and are highly toxic to them.”
The Northwestern team discovered that their nanotherapy can effectively capture these harmful proteins before they aggregate into the toxic structures capable of penetrating neurons. Once trapped, the proteins harmlessly degrade in the body.
A Sweet Solution to a Complex Problem
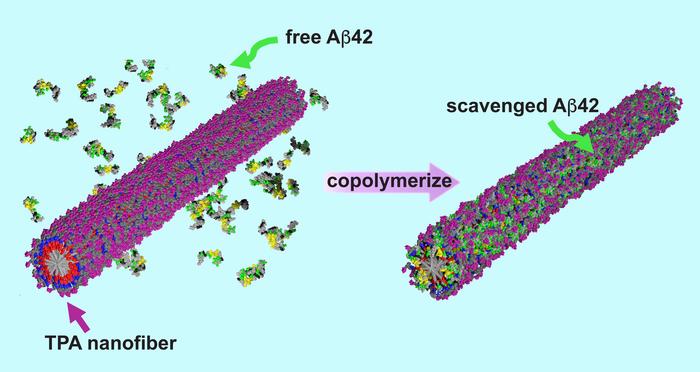
The researchers developed their therapy using peptide amphiphiles – a class of molecules already found in well-known pharmaceuticals like Ozempic. What makes this treatment unique is the addition of trehalose, a natural sugar found in plants, fungi, and insects.
“Trehalose is naturally occurring in plants, fungi and insects,” explained Zijun Gao, a Ph.D. candidate in Stupp’s laboratory and the paper’s first author. “It protects them from changing temperatures, especially dehydration and freezing. Others have discovered trehalose can protect many biological macromolecules, including proteins. So, we wanted to see if we could use it to stabilize misfolded proteins.”
How the Nanotherapy Works
When added to water, the peptide amphiphiles self-assembled into nanofibers coated with trehalose. Surprisingly, researchers found that the trehalose actually destabilized these nanofibers – which turned out to be beneficial.
The resulting unstable nanofibers actively seek out and bond with toxic amyloid-beta proteins implicated in Alzheimer’s disease. Once bonded, the nanofibers permanently incorporate these proteins into their structure, effectively neutralizing them.
“Then, it’s no longer a peptide amphiphile fiber anymore,” Stupp said. “But a new hybrid structure comprising both the peptide amphiphile and the amyloid-beta protein. That means the nasty amyloid-beta proteins, which would have formed amyloid fibers, are trapped. They can no longer penetrate the neurons and kill them. It’s like a clean-up crew for misfolded proteins.”
Promising Results for Neural Survival
Laboratory tests using human neurons derived from stem cells showed remarkable results. When neurons were exposed to toxic amyloid-beta proteins:
- Untreated neurons died in significant numbers
- Neurons treated with the trehalose-coated nanofibers showed dramatically improved survival
- Both motor neurons and cortical neurons were protected
- The therapy effectively prevented formation of the most toxic early-stage amyloid structures
Microscope images clearly show the difference – untreated neurons exposed to amyloid-beta proteins primarily appear red (dead), while treated neurons remain predominantly green (alive).
A Novel Mechanism with Safety Advantages
This approach differs significantly from most current Alzheimer’s therapies, which often target fully-formed amyloid plaques or attempt to reduce inflammation. By intervening earlier in the disease process, this treatment could potentially prevent damage before it occurs.
The therapy also offers potential safety advantages. “The advantage of peptide-based drugs is that they degrade into nutrients,” Stupp noted. “The molecules in this novel therapeutic concept break down into harmless lipids, amino acids and sugars. That means there are fewer adverse side effects.”
With an estimated 50 million people worldwide suffering from neurodegenerative disorders according to the World Health Organization, the need for effective treatments is urgent. While current therapies offer limited relief, this new approach could potentially change how these diseases are treated.
What might this mean for patients in the future? Stupp suggests the therapy would likely work best at earlier disease stages, possibly in combination with other treatments. “Our therapy might work best when targeting diseases at an earlier stage — before aggregated proteins enter cells,” he said. “But it’s challenging to diagnose these diseases at early stages. So, it could be combined with therapies that target later-stage symptoms of the disease. Then, it could be a double whammy.”
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!