When we dream, our brains are filled with noisy electrical activity that looks nearly identical to that of the awake brain.
But UC Berkeley researchers have pulled a signal out of the noise that uniquely defines dreaming, or REM sleep, potentially making it easier to monitor people with sleep disorders, as well as unconscious coma patients or those under anesthesia.
Each year, hundreds of thousands of people undergo overnight studies to diagnose problems with their sleep, most of them hooked up to an electroencephalogram (EEG) to monitor brain activity as they progress from wakefulness to deep, slow-wave sleep and on into REM sleep. But EEGs alone can not tell whether a patient is awake or dreaming: Doctors can only distinguish REM sleep by recording rapid eye movement — hence, the name — and muscle tone, since our bodies relax in a general paralysis to prevent us from acting out our dreams.
“We really now have a metric that precisely tells you when you are in REM sleep. It is a universal metric of being unconscious,” said Robert Knight, UC Berkeley professor of psychology and neuroscience and senior author of a paper describing the research that was published July 28 in the online journal eLife.
“These new findings show that, buried in the electrical static of the human brain, there is something utterly unique — a simple signature,” said co-author and sleep researcher Matthew Walker, UC Berkeley professor of psychology and neuroscience. “And if we measure that simple electrical signature, for the first time, we can precisely determine exactly what state of consciousness someone is experiencing — dreaming, wide awake, anesthetized or in deep sleep.”
The ability to distinguish REM sleep by means of an EEG will allow doctors to monitor people under anesthesia during surgery to explore how narcotic-induced unconsciousness differs from normal sleep — a still-unsettled question. That’s the main reason first author Janna Lendner, a medical resident in anesthesiology, initiated the study.
“We often tell our patients that, ‘You will go to sleep now,’ and I was curious how much these two states actually overlap,” said Lendner, a UC Berkeley postdoctoral fellow in her fourth year of residency in anesthesiology at the University Medical Center in Tübingen, Germany. “Anesthesia can have some side effects. If we learn a little bit about how they overlap — maybe anesthesia hijacks some sleep pathways — we might be able to improve anesthesia in the long run.”
Sleep soothes the brain
Sleep, as Walker wrote in his 2017 book, “Why we Sleep,” “enriches a diversity of functions, including our ability to learn, memorize, and make logical decisions and choices. Benevolently servicing our psychological health, sleep recalibrates our emotional brain circuits, allowing us to navigate next-day social and psychological challenges with cool-headed composure.”
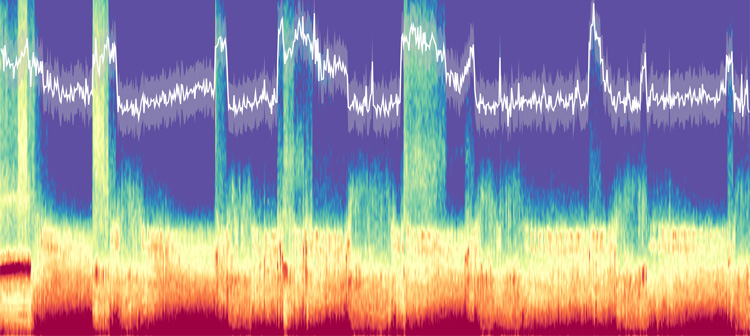
Brain activity recorded by three frontal scalp EEG electrodes during a night of sleep. In white, the time-resolved spectral slope tracks the underlying changes in arousal level. (Image by Janna Lendner et al, CC BY 4.0)
Disrupted sleep interferes with all of this, increasing the risk of medical, psychiatric and neurological diseases.
Most sleep research focuses on the synchronized, rhythmic waves that flow through the neural network of the brain, from the slow waves that signal deep sleep, typically in the first few hours of the night, to the higher frequency waves typical of dream sleep. These waves pop out above a lot of general activity, also called the 1/f, that has typically been dismissed as noise and ignored.
But Knight and his lab have been looking at this “noise” for a decade and found that it contains useful information about the state of the brain. In 2015, for example, he and Bradley Voytek, a former doctoral student now on the faculty at UC San Diego, discovered that the amount of high frequency activity increases with age. Lendner has now found that a faster drop-off of high-frequency activity, relative to low-frequency activity, is a unique signature of REM sleep.
“There is this background activity, which is not rhythmic, and we have overlooked that for quite a long time,” Lendner said. “Sometimes, it has been called noise, but it is not noise; it carries a lot of information, also about the underlying arousal level. This measure makes it possible to distinguish REM sleep from wakefulness by looking only at the EEG.”
Since slow waves are associated with inhibition of activity in the brain, while high frequency activity — like that found during wakefulness — is associated with excitatory behavior, the sharper drop-off may be an indication that many activities in the brain, including those related to muscle movement, are being tamped down during REM sleep.
The new measure quantifies the relationship of brain activity at different frequencies — how much activity there is at frequencies from about 1 cycle per second to 50 cycles per second — and determines the slope, that is, how fast the spectrum drops. This 1/f “drop-off” is sharper in REM sleep than in wakefulness or when under anesthesia.
Lendner found this characteristic measure in the nighttime brain activity of 20 people recorded via EEG scalp electrodes in Walker’s UC Berkeley sleep lab and in 10 people who had electrodes placed in their brains to search for the causes of epilepsy as a necessary prologue to brain surgery to alleviate seizures.
She also recorded brain activity in 12 epilepsy patients and 9 other patients undergoing spinal surgery with the common general anesthetic Propofol.
Lendner is now reviewing brain recordings from coma patients to see how their brain activity varies over the course of a day and whether the 1/f drop-off can be used to indicate the likelihood of emergence from coma.
“More importantly, I think it is another metric for evaluating states of coma,” Knight said. “1/f is very sensitive. It could resolve, for instance, if someone was in a minimally conscious state, and they are not moving, and whether they are more alert than you think they are.”
The work was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft), National Institute of Neurological Disorders and Stroke and National Institute of Mental Health. Other co-authors of the eLife paper are Randolph Helfrich of the University Medical Center in Tübingen, Bryce Mander and Jack Lin of UC Irvine and Luis Romundstad and Pal Larsson of the University of Oslo Medical Center. Knight, Walker and Lendner are members of UC Berkeley’s Helen Wills Neuroscience Institute.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!


