Researchers have developed a novel antibiotic that effectively fights dangerous gram-positive bacteria, including those responsible for flesh-eating diseases. The compound, tested successfully in mice, could lead to a new class of antibiotics to combat increasingly resistant infections.
A Serendipitous Discovery in the Battle Against Superbugs
Scientists at Washington University School of Medicine in St. Louis have created a compound that clears bacterial infections in mice, including those that can cause rare but potentially fatal “flesh-eating” illnesses. This new antibiotic, part of a family called GmPcides, targets gram-positive bacteria – a group that includes pathogens responsible for drug-resistant staph infections and toxic shock syndrome.
The research, published in Science Advances, stems from a collaboration between microbiologists Scott Hultgren and Michael Caparon, and chemist Fredrik Almqvist from the University of Umeå in Sweden. Initially aiming to prevent bacterial films on urinary catheters, the team stumbled upon the compound’s broader infection-fighting properties.
“All of the gram-positive bacteria that we’ve tested have been susceptible to that compound,” said Caparon, the study’s co-senior author. “That includes enterococci, staphylococci, streptococci, C. difficile, which are the major pathogenic bacteria types.”
Promising Results Against Flesh-Eating Disease
The researchers focused their study on Streptococcus pyogenes, a bacterium responsible for 500,000 deaths globally each year, including cases of necrotizing fasciitis, commonly known as flesh-eating disease. In mouse models, animals treated with GmPcide showed remarkable improvements:
– Less weight loss
– Smaller infection-related ulcers
– Faster recovery from infection
Surprisingly, the compound not only reduced bacterial virulence but also appeared to accelerate healing of damaged skin areas post-infection.
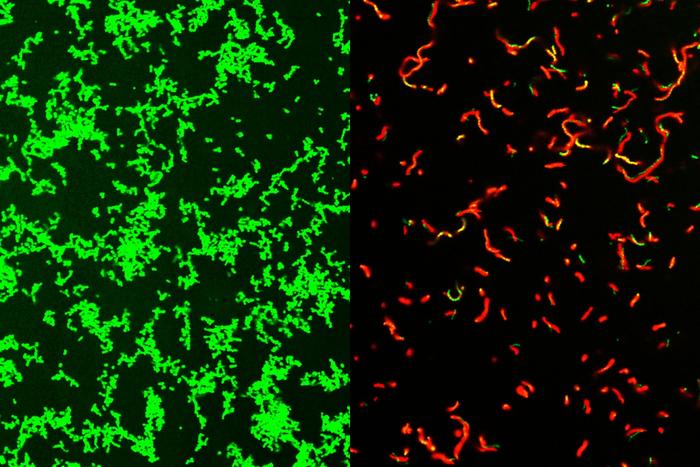
While the exact mechanism remains unclear, microscopic examination revealed that GmPcides significantly affect bacterial cell membranes. “We know that within five to ten minutes of treatment with GmPcide, the membranes start to become permeable and allow things that normally should be excluded to enter into the bacteria, which suggests that those membranes have been damaged,” Caparon explained.
This membrane disruption may interfere with the bacteria’s harmful functions and make them more vulnerable to the host’s immune response.
Why it matters: As antibiotic resistance continues to grow, new classes of antimicrobials are crucial for combating dangerous infections. GmPcides offer hope for treating increasingly resistant gram-positive bacterial infections, potentially saving lives and limbs from devastating diseases like necrotizing fasciitis.
The compound’s broad-spectrum activity and apparent ability to enhance healing could make it a valuable tool in the medical arsenal. Additionally, early experiments suggest that bacteria may be less likely to develop resistance to GmPcides compared to traditional antibiotics.
However, the road to clinical use remains long. The compound has been patented and licensed to QureTech Bio, a company in which the researchers have a stake. Further pharmaceutical development and clinical trials will be necessary before GmPcides could potentially reach the market.
Hultgren emphasized the importance of collaborative, interdisciplinary science in addressing complex health challenges: “Bacterial infections of every type are an important health problem, and they are increasingly becoming multi-drug resistant and thus harder to treat. Interdisciplinary science facilitates the integration of different fields of study that can lead to synergistic new ideas that have the potential to help patients.”
As research continues, the development of GmPcides represents a promising step forward in the ongoing battle against antibiotic-resistant infections. Future studies will likely explore the compound’s effectiveness against a wider range of pathogens and its potential applications in various clinical settings.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!