Summary: A new study reveals that glioblastoma tumors regrow from scar tissue left behind after treatment, offering potential new targets for combination therapies to prevent relapse.
Estimated reading time: 7 minutes
Researchers at Ludwig Cancer Research have made a significant discovery in the fight against glioblastoma multiforme (GBM), the most aggressive form of brain cancer in adults. Their study, published in Cancer Cell, shows that recurrent GBM tumors emerge from the fibrous scars left behind after initial treatments such as radiotherapy, surgery, and immunotherapy.
This finding sheds new light on why GBM is so difficult to treat and offers promising avenues for developing more effective therapies. With the average life expectancy for GBM patients remaining at around 14 months after diagnosis, this research could have far-reaching implications for improving patient outcomes.
Why it matters: GBM is notoriously resistant to treatment, with tumors often recurring despite aggressive interventions. Understanding the mechanisms behind this recurrence is crucial for developing more effective therapies. This research not only explains why current treatments often fail but also points to new strategies for preventing tumor regrowth.
The Scar Tissue Connection
The study, led by Johanna Joyce of Ludwig Lausanne, builds on earlier research from 2016 that examined strategies to overcome resistance to immunotherapy in GBM. That work, published in Science, revealed a curious pattern: recurring tumors always regrew next to scars formed at the original tumor site.
In the current study, Joyce and her team confirmed that this phenomenon occurs in human patients as well. They found that fibrotic scarring happens not just after immunotherapy, but also following surgical removal of tumors and radiotherapy.
“We’ve identified fibrotic scarring as a key source of GBM resurgence following therapy, showing how it creates a protective niche for the regrowth of the tumor,” said Joyce.
Unraveling the Mechanism
To understand how these scars contribute to tumor recurrence, the researchers employed a suite of advanced technologies, including:
- Single-cell gene expression analysis
- Comprehensive protein analysis of tissues
- A novel AI-powered imaging technique called hyperplexed immunofluorescence imaging (HIFI)
These methods allowed the team to create detailed maps of the tumor microenvironment and observe the formation of fibrotic scars at a cellular level.
Spencer Watson, a co-author of the study, explained their findings: “Applied together, these advanced methods allowed us to see exactly how fibrotic scars form. They revealed that the fibrosis serves as a kind of protective cocoon for residual cancer cells and pushes them into a dormant state in which they are largely resistant to therapy. We found that it also shields them from surveillance and elimination by the immune system.”
From Blood Vessels to Fibrotic Scars
The researchers discovered that cells associated with tumor blood vessels transform into fibroblast-like cells following treatment. These cells, which they named perivascular-derived fibroblast-like (PDFL) cells, spread across the area previously occupied by the tumor and create fibrotic scars.
The activation of these PDFL cells is particularly influenced by neuroinflammation and immune factors called cytokines, especially one known as transforming growth factor-β (TGF-β).
Targeting Fibrotic Scarring for Better Outcomes
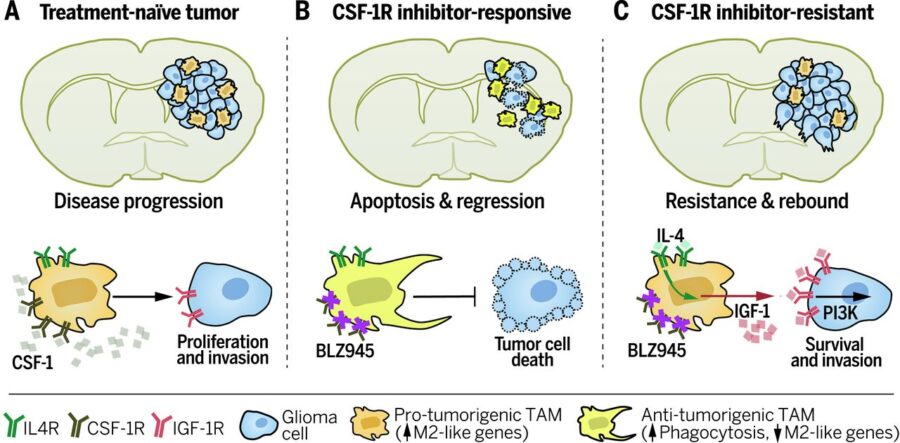
Armed with this new understanding, the team devised a treatment strategy to target fibrotic scarring. They combined existing drugs to block TGF-β signaling and suppress neuroinflammation with a therapy that inhibits colony stimulating factor-1 receptor (CSF-1R), which had shown promise in earlier studies.
“To see if targeting fibrotic scarring could improve therapeutic outcomes for GBM, we devised a treatment regimen using existing drugs to block TGF-β signaling and suppress neuroinflammation in combination with CSF-1R inhibition and evaluated it in preclinical trials using mouse models of GBM,” Joyce explained. “We also timed these additional treatments to coincide with the period of maximal PDFL activation identified by our studies.”
The results were encouraging. The combination therapy inhibited fibrotic scarring, reduced the number of surviving tumor cells, and extended the survival of treated mice compared to control groups.
Looking Ahead: Implications for GBM Treatment
This research opens up new possibilities for improving GBM treatment. By targeting the formation of fibrotic scars, it may be possible to significantly enhance the effectiveness of existing therapies like surgery, radiation, and immunotherapy.
The study authors suggest that further research could yield even better drug targets for combination therapies. As clinical trials for CSF-1R inhibition in glioma patients are already underway, these findings could have immediate relevance for improving treatment strategies.
While this research represents a significant step forward, it’s important to note that translating these findings into effective treatments for humans will require additional studies and clinical trials. However, the identification of fibrotic scarring as a key factor in GBM recurrence provides a promising new direction for research and treatment development.
Quiz:
- What type of cancer did this study focus on?
- What cellular transformation contributes to the formation of fibrotic scars in GBM?
- Which cytokine was identified as particularly important in activating cells that form fibrotic scars?
Answer Key:
- Glioblastoma multiforme (GBM)
- Blood vessel-associated cells transforming into perivascular-derived fibroblast-like (PDFL) cells
- Transforming growth factor-β (TGF-β)
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!