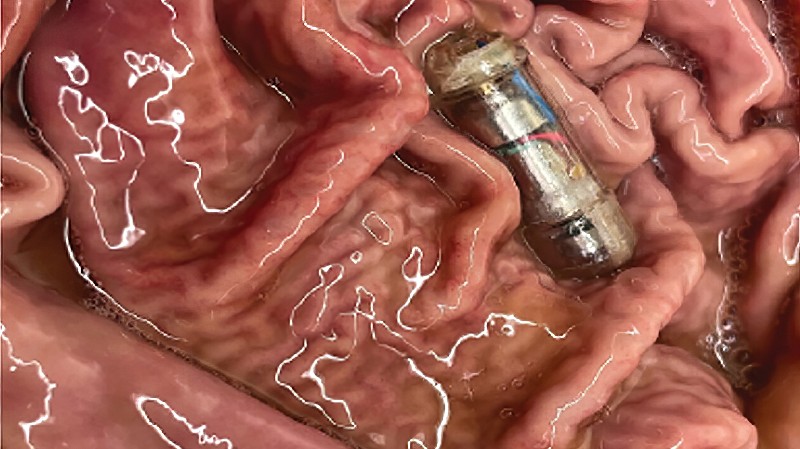
Obesity interventions, such as gastric bypass surgery, can alter the signaling of the vagal nerve, which plays a crucial role in regulating digestion. In addition to traditional obesity interventions, new weight-management medications, such as Wegovy, are becoming increasingly popular options for patients with obesity since they are non-invasive and require minimal lifestyle modifications. Developing a variety of non-invasive, convenient weight-management options for patients with obesity is essential to help reduce comorbidities such as diabetes, hypertension, cancer and heart disease.
Investigators from Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, and MIT have created a pill-size device, called the vibrating ingestible bioelectronic stimulator (VIBE),that safely travels through the gastrointestinal tract and takes up space in the stomach by vibrating upon contact with gastric fluid, stimulating vagal nerve receptors and creating the sensation of fullness. The team placed the device in the stomach of swine and saw an average of 31% reduction in food intake. Additionally, the research team found that the device remained in the digestive system, on average, for 30 minutes, suggesting that it should be swallowed before meals. While additional preclinical studies are needed, the findings suggest that the ingestible device could be an effective and sustainable method to prevent weight gain and reduce the number of calories consumed during meals.
“Our study demonstrates the effectiveness of a low-cost, non-invasive intervention to reduce food intake and calorie consumption. The device functions effectively in the stomach and induces satiety,” said corresponding author Giovanni Traverso, MB, PhD, MBBCH, a gastroenterologist in the Division of Gastroenterology, Hepatology and Endoscopy at the Brigham. “The device has the potential to revolutionize therapeutic options for patients with obesity. However, future studies will need to explore the physiological effects of the device before it’s available for patients.”
Read more in Science Advances.
Obesity interventions, such as gastric bypass surgery, can alter the signaling of the vagal nerve, which plays a crucial role in regulating digestion. In addition to traditional obesity interventions, new weight-management medications, such as Wegovy, are becoming increasingly popular options for patients with obesity since they are non-invasive and require minimal lifestyle modifications. Developing a variety of non-invasive, convenient weight-management options for patients with obesity is essential to help reduce comorbidities such as diabetes, hypertension, cancer and heart disease.
Investigators from Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, and MIT have created a pill-size device, called the vibrating ingestible bioelectronic stimulator (VIBE),that safely travels through the gastrointestinal tract and takes up space in the stomach by vibrating upon contact with gastric fluid, stimulating vagal nerve receptors and creating the sensation of fullness. The team placed the device in the stomach of swine and saw an average of 31% reduction in food intake. Additionally, the research team found that the device remained in the digestive system, on average, for 30 minutes, suggesting that it should be swallowed before meals. While additional preclinical studies are needed, the findings suggest that the ingestible device could be an effective and sustainable method to prevent weight gain and reduce the number of calories consumed during meals.
“Our study demonstrates the effectiveness of a low-cost, non-invasive intervention to reduce food intake and calorie consumption. The device functions effectively in the stomach and induces satiety,” said corresponding author Giovanni Traverso, MB, PhD, MBBCH, a gastroenterologist in the Division of Gastroenterology, Hepatology and Endoscopy at the Brigham. “The device has the potential to revolutionize therapeutic options for patients with obesity. However, future studies will need to explore the physiological effects of the device before it’s available for patients.”
Read more in Science Advances.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!