A research team led by Dr. Yoonseok Choi from the Korea Institute of Energy Research (KIER), in collaboration with Professor WooChul Jung from KAIST and Professor Beom-Kyung Park from Pusan National University, has developed a catalyst coating technology that significantly enhances the performance of solid oxide fuel cells (SOFCs) in just 4 minutes. This breakthrough could potentially drive the hydrogen economy forward by improving the efficiency and clean energy capabilities of fuel cells.
Solid oxide fuel cells (SOFCs) are gaining attention for their high power generation efficiency and ability to use various fuels such as hydrogen, biogas, and natural gas. However, the performance of SOFCs is largely determined by the kinetics of the oxygen reduction reaction (ORR) occurring at the air electrode (cathode), which is slower than the reaction at the fuel electrode (anode), thus limiting the overall reaction rate.
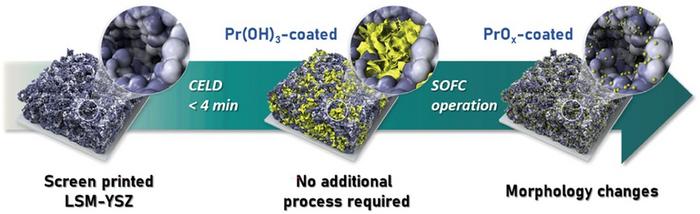
Enhancing Performance of Widely Used LSM-YSZ Composite Electrode
Instead of developing new air electrode materials, the research team focused on enhancing the performance of the LSM-YSZ composite electrode, a material widely used in the industry due to its excellent stability. They developed a coating process for applying nanoscale praseodymium oxide (PrOx) catalysts on the surface of the composite electrode, which actively promotes the oxygen reduction reaction.
The researchers introduced an electrochemical deposition method that operates at room temperature and atmospheric pressure, requiring no complex equipment or processes. By immersing the composite electrode in a solution containing praseodymium (Pr) ions and applying an electric current, a precipitate forms and uniformly coats the electrode. This coating layer undergoes a drying process, transforming into an oxide that remains stable and effectively promotes the oxygen reduction reaction of the electrode in high-temperature environments. The entire coating process takes only 4 minutes.
Tenfold Reduction in Polarization Resistance and Threefold Increase in Power Density
The research team elucidated the mechanism by which the coated nano-catalyst promotes surface oxygen exchange and ionic conduction, providing fundamental evidence that the catalyst coating method can address the low reaction rate of the composite electrode.
By operating the developed catalyst-coated composite electrode and the conventional composite electrode for over 400 hours, the team observed that the polarization resistance was reduced tenfold. Additionally, the SOFC using this coated electrode exhibited a peak power density three times higher (142 mW/cm² → 418 mW/cm²) than that of the uncoated case, at 650 degrees Celsius. This represents the highest performance reported for SOFCs using LSM-YSZ composite electrodes in literature.
Dr. Yoonseok Choi, co-corresponding author, stated, “The electrochemical deposition technique we developed is a post process that does not significantly impact the existing manufacturing process of SOFCs. This makes it economically viable for introducing oxide nano-catalysts, enhancing its industrial applicability.” He added, “We have secured a core technology that can be applied not only to SOFCs but also to various energy conversion devices, such as high-temperature electrolysis (SOEC) for hydrogen production.”
The research findings were published in Advanced Materials, a world-renowned journal in materials science, and the study was conducted with support from the Ministry of Trade, Industry and Energy’s Core Technology Development Program for New and Renewable Energy and the Ministry of Science and ICT’s Individual Basic Research Program.
For more information, explore the following resources:
- The original research article in Advanced Materials
- Korea Institute of Energy Research (KIER)
- KAIST Department of Materials Science and Engineering
- Pusan National University Department of Materials Science and Engineering
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!