Summary: Brazilian researchers have developed an innovative method using magnetic nanoparticles to capture and remove micro- and nanoplastics from water. The technique, which mimics mussel adhesion properties, could help address one of today’s most pressing environmental challenges while potentially enabling plastic recycling.
Journal: Micron, September 18, 2024, DOI: 10.1016/j.micron.2024.103722 | Reading time: 4 minutes
The Invisible Threat in Our Water
In our modern world, an invisible menace lurks in every drop of water we encounter. Micro- and nanoplastics, tiny fragments of synthetic materials, have become ubiquitous in our environment – from the deepest oceans to the water we drink. These particles, which can be as small as one-thousandth of a millimeter, pose a particularly insidious threat because they can bypass biological barriers and accumulate in vital organs.
“Nanoparticles aren’t visible to the naked eye or detectable using conventional microscopes, so they’re very hard to identify and remove from water treatment systems,” explains Henrique Eisi Toma, professor at the University of São Paulo’s Institute of Chemistry and lead researcher of a groundbreaking new study.
Nature-Inspired Solution
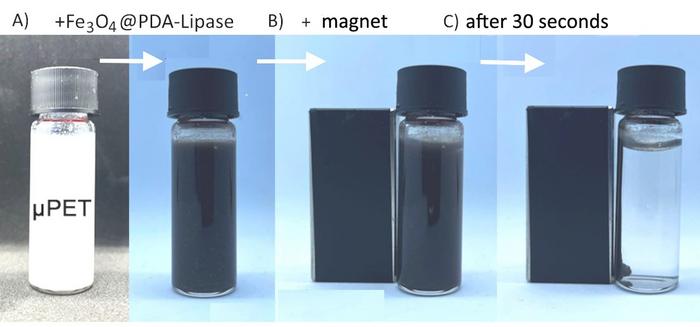
The research team at the University of São Paulo has developed an elegant solution that draws inspiration from an unlikely source: mussels. Their method employs magnetic nanoparticles coated with polydopamine, a polymer that mimics the remarkable adhesive properties of mussels.
“Polydopamine is a substance that mimics the adhesive properties of mussels, which cling very tenaciously to many surfaces. It adheres firmly to fragments of plastic in water and enables the magnetic nanoparticles to capture them. This undesirable material can then be removed from the water with a magnet,” Toma said.
Beyond Removal: A Sustainable Approach
The team’s innovation goes beyond simply removing plastic waste. By incorporating specific enzymes like lipase, the system can actually break down common plastics such as PET (polyethylene terephthalate) into their basic components, opening the door for recycling.
The process has shown particular promise for water treatment systems, offering a potential solution to a problem that has long frustrated environmental scientists. The research team used hyperspectral Raman microscopy to monitor the plastic sequestration and degradation in real time, confirming the method’s effectiveness.
Glossary:
– Polydopamine: A polymer derived from dopamine that mimics mussel adhesive properties
– Nanoplastics: Plastic particles smaller than 0.001 millimeter in size
– Microplastics: Plastic fragments up to 1 millimeter in size
– Hyperspectral Raman microscopy: An imaging technique that can monitor chemical processes in real-time
– PET: Polyethylene terephthalate, a common plastic used in bottles and packaging
Quiz:
1. What material inspired the solution for capturing microplastics?
Answer: Mussels’ adhesive properties
2. What type of nanoparticles are used in this new method?
Answer: Magnetic nanoparticles coated with polydopamine
3. What enzyme is used to break down PET plastic?
Answer: Lipase
4. How are the plastic particles removed from water once captured?
Answer: Using a magnet
Enjoy this story? Get our newsletter! https://scienceblog.substack.com/
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!