In a surprising twist that could help combat the growing crisis of antibiotic resistance, researchers have discovered that some bacteria pay a hidden price for their ability to survive antibiotics. The secret lies in how these resistant bacteria struggle to manage their internal magnesium supplies, potentially opening a new front in the fight against drug-resistant infections.
Journal: Science Advances, November 15, 2024, DOI: 10.1126/sciadv.adq5249 | Reading time: 4 minutes
The Magnesium Mystery
Professor Gürol Süel and colleagues at the University of California San Diego posed a deceptively simple question: If antibiotic-resistant bacteria have such an advantage, why don’t they completely take over bacterial populations? Their investigation revealed an unexpected answer – resistant bacteria’s survival comes at a significant metabolic cost.
“We discovered an Achilles heel of antibiotic resistant bacteria,” said Süel, from UC San Diego’s Department of Molecular Biology. “We can take advantage of this cost to suppress the establishment of antibiotic resistance without drugs or harmful chemicals.”
A Molecular Tug-of-War
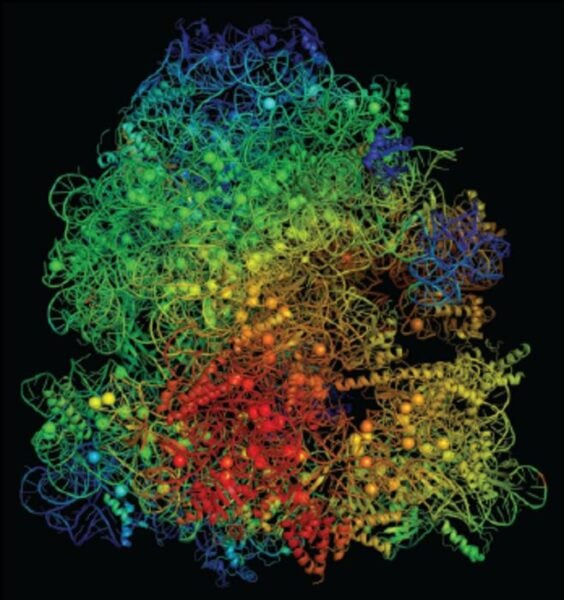
The research team found that antibiotic-resistant bacteria face an internal resource crisis. Their modified ribosomes – cellular machines that produce proteins – require more magnesium to function compared to normal bacteria. This creates a molecular tug-of-war between ribosomes and ATP (the cell’s energy currency) for limited magnesium resources.
Working with colleagues at Arizona State University and the Universitat Pompeu Fabra in Spain, the researchers studied a specific bacterial strain called Bacillus subtilis. Using atomic-scale modeling, they discovered that mutant ribosomes that provide antibiotic resistance compete excessively for magnesium ions, creating an internal resource shortage that hampers their growth.
Practical Implications
This discovery could lead to new strategies for fighting antibiotic-resistant infections without using traditional antibiotics. Scientists might be able to target bacteria’s magnesium usage, selectively inhibiting resistant strains while leaving beneficial bacteria unharmed.
“We are running out of effective antibiotics and their rampant use over the decades has resulted in antibiotics being spread across the globe, from the arctic to the oceans and our groundwater,” Süel explained. “Drug-free alternatives to treating bacterial infections are needed.”
Key Terms
- Ribosome: Cellular machinery responsible for protein synthesis
- ATP: Adenosine triphosphate, the primary energy carrier in cells
- Magnesium ions: Essential minerals that help stabilize cellular structures
- Bacillus subtilis: A type of bacteria commonly used in research
Test Your Knowledge
1. What is the key disadvantage discovered in antibiotic-resistant bacteria?
They require more magnesium for their ribosomes to function properly, creating an internal resource shortage.
2. Why is this discovery significant for treating infections?
It suggests a way to combat antibiotic-resistant bacteria without using traditional antibiotics by targeting their magnesium usage.
3. What creates the “molecular tug-of-war” in resistant bacteria?
The competition between ribosomes and ATP molecules for limited magnesium resources.
4. Who led this research and at which institution?
Professor Gürol Süel at the University of California San Diego.
Enjoy this story? Subscribe to our newsletter at scienceblog.substack.com
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!