Japanese researchers have developed a precise genetic technique that can remove the extra chromosome responsible for Down syndrome in human cells, potentially opening new avenues for treating the most common genetic cause of cognitive impairment.
The groundbreaking study, https://academic.oup.com/pnasnexus/article/4/2/pgaf022/8016019?login=falses, demonstrates how a modified version of the gene-editing tool CRISPR-Cas9 can selectively target and eliminate the additional copy of chromosome 21 that causes Down syndrome, effectively restoring normal cellular function in both stem cells and specialized human cells.
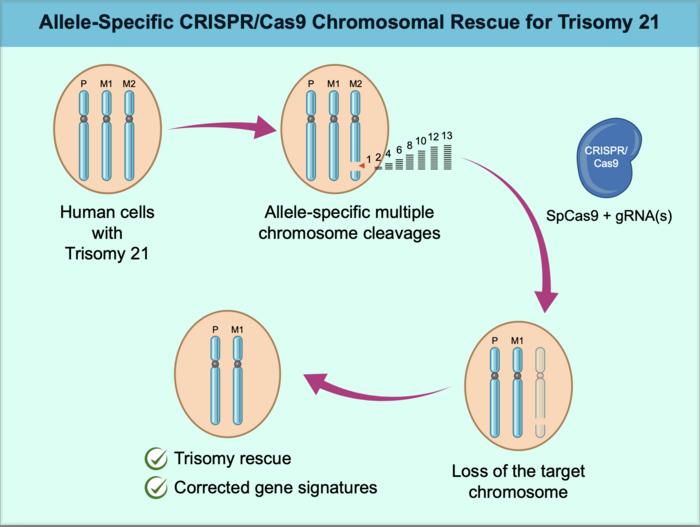
The research team developed a new approach that efficiently targets and removes specific chromosomes, explained lead researcher Ryotaro Hashizume and colleagues from Mie University Graduate School of Medicine in Japan. Their method improves upon previous strategies by using a more comprehensive and precise targeting system.
Down syndrome affects approximately one in every 700 live births globally, resulting from an extra copy of chromosome 21. While extensive research has illuminated the condition’s clinical features and genetic causes over the past half-century, few studies have tackled its fundamental cause – the presence of that extra chromosome.
The research team’s novel approach uses a precisely targeted version of CRISPR to identify and cut specific DNA sequences found only on the extra chromosome. This specificity is crucial, as it allows them to remove just the extra copy while leaving the normal pair of chromosomes intact.
In laboratory tests, the technique successfully reduced the number of chromosomes from three to the normal two in both stem cells and skin cells derived from a person with Down syndrome. The correction rate reached nearly 17% in stem cells when researchers also temporarily suppressed certain DNA repair mechanisms.
Notably, cells that had the extra chromosome removed showed significant improvements in several areas. The treated cells exhibited enhanced growth rates and reduced production of harmful reactive oxygen species – a known issue in cells with Down syndrome. Gene activity patterns also shifted closer to those seen in cells without Down syndrome.
The researchers found that correcting the chromosome number restored normal gene activity patterns and improved cellular function. Importantly, their technique worked not only in stem cells but also in specialized cells that don’t actively divide.
While the research marks a significant technical achievement, the path to any potential clinical applications remains long and complex. The current method still results in some unintended genetic modifications in cells where the extra chromosome isn’t successfully removed, and extensive safety testing would be needed before any human trials could begin.
The study also raises important ethical considerations about potential future treatments for Down syndrome, a condition that many advocate groups emphasize should not be viewed as something that needs to be “cured.”
The researchers acknowledge these challenges while highlighting the scientific significance of their work. They anticipate that their allele-specific approach “will lay the groundwork for more sophisticated medical interventions targeting trisomy 21.”
Looking ahead, the team suggests several areas for improvement, including developing methods that don’t rely on making cuts in the DNA and creating more efficient delivery systems for potential future therapeutic applications.
This research represents a significant step forward in understanding how to manipulate chromosomes with precision, potentially opening new pathways for treating not just Down syndrome but other chromosomal conditions as well.
If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!