The symptoms of type 2 diabetes can be induced by a misfolded form of a pancreatic protein and possibly be transmitted by a mechanism similar to prion diseases such as Creutzfeldt-Jakob disease or bovine spongiform encephalopathy (mad cow disease), according to researchers from McGovern Medical School at The University of Texas Health Science Center at Houston (UTHealth).
The findings were reported today in a paper published in The Journal of Experimental Medicine.
The Centers for Disease Control and Prevention estimates that 29 million Americans suffer from type 2 diabetes, a condition in which the body is unable to regulate blood glucose levels using the hormone insulin. Although the disease has been linked to a variety of genetic and environmental risk factors, what causes type 2 diabetes is still not completely understood.
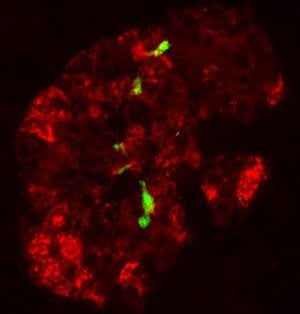
More than 90 percent of type 2 diabetes patients show abnormal protein deposits in their pancreatic islets that are aggregates of a misfolded form of a protein called islet amyloid polypeptide (IAPP). The precise role of these IAPP aggregates in type 2 diabetes is unclear, but they may damage and kill the pancreatic beta cells that secrete insulin in response to elevated blood glucose levels. In this respect, type 2 diabetes could be similar to other diseases caused by misfolded protein aggregates, such as Alzheimer’s disease, Parkinson’s disease and prion disorders.
“Until now, this concept has not been considered,” said Claudio Soto, Ph.D., senior author, professor in the Department of Neurology and the director of the George and Cynthia Mitchell Center for Alzheimer’s Disease and Related Brain Disorders at UTHealth. “Our data therefore opens up an entirely new area of research with profound implications for public health. This prion-like mechanism may play a key role in the spreading of the pathology from cell to cell or islet to islet during the progression of type 2 diabetes.”
A key feature of these diseases is that a small number of misfolded protein aggregates can serve as “seeds” that induce the misfolding of additional proteins until they form large aggregates capable of damaging the cells. In the case of prion diseases, these seeds can even be transmitted from one individual to another but Soto said they don’t know yet if that is the case in diabetes.
Soto and his colleagues found that injecting small amounts of misfolded IAPP aggregates induced the formation of protein deposits in the pancreases of mice expressing human IAPP. Within weeks, these mice developed several symptoms associated with type 2 diabetes, including a loss of pancreatic beta cells and elevated blood glucose levels. Small amounts of misfolded IAPP could also induce the formation and accumulation of large IAPP aggregates in pancreatic islets isolated from healthy human donors.
Misfolded IAPP can therefore induce protein aggregation and disease similarly to infectious prion proteins, but Soto said that it is much too soon to conclude that type 2 diabetes can be transmitted between individuals.
“Considering the experimental nature of the models and conditions utilized in this study, the results should not be extrapolated to conclude that type 2 diabetes is a transmissible disease in humans without additional studies,” Soto said.
Co-first authors of the paper are Abhisek Mukherjee, Ph.D., postdoctoral researcher at McGovern Medical School at UTHealth; Diego A. Morales Scheihing, Ph.D., program manager, Department of Neurology, McGovern Medical School; and Natalia Salvadores, Ph.D.

I’ve read that an unusual amino acid called BMAA sometimes substitutes for l-serine (the human pathway for assembling proteins cannot tell them apart), and causes an unexpected kink in the protein that can lead to misfolding.